| In vitro: |
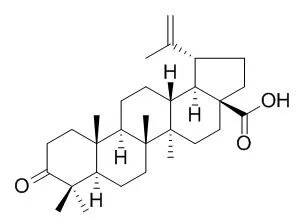
| Bioorg Med Chem. 2014 Jul 1;22(13):3292-300. | | Synthesis of triterpenoid triazine derivatives from allobetulone and betulonic acid with biological activities.[Pubmed: 24844757] | The synthetic transformation and modification of natural products with the aim to improve the biological properties is an area of current interest. The triterpenoids betulin and betulinic acid are very abundant in nature and now are commercially available.
METHODS AND RESULTS:
In our study, starting from betulin and betulinic acid, we obtained allobetulone and Betulonic acid in a few synthetic steps. The ketone function at the A-ring was used as the starting point for the synthesis of a series of 1,2,4-triazine-fused triterpenoids. The alkylation and Liebeskind-Srogl coupling were used for further substitution of 1,2,4-triazines, and the intramolecular hetero Diels-Alder reaction leads to interesting fused thienopyridine derivatives. All new compounds were tested for their cytostatic activities against murine leukemia L1210, human cervix carcinoma HeLa and human lymphoblast CEM tumor cells.
CONCLUSIONS:
The results show that some triterpenoid triazine Betulonic acid derivatives have a promising cytostatic activity in vitro and could be used as potential leads for the development of new type of anti-cancer agents. Several compounds were also endowed with anti-HCMV activity in the low micromolar range. | | Fitoterapia. 2003 Jul;74(5):489-92. | | Antiviral activity of betulin, betulinic and betulonic acids against some enveloped and non-enveloped viruses.[Pubmed: 12837369] |
METHODS AND RESULTS:
Antiviral properties of betulin, betulinic and Betulonic acids were investigated in cell cultures infected with herpes simplex type I, influenza FPV/Rostock and ECHO 6 viruses.
CONCLUSIONS:
All studied triterpenes were active against herpes simplex virus. Betulin and especially betulinic acid also suppressed ECHO 6 virus reproduction. | | Eur J Med Chem . 2015;96:58-65. | | Synthesis and biological evaluation of betulonic acid derivatives as antitumor agents[Pubmed: 25874331] | | Abstract
Structural modification was performed at the C-28 position of Betulonic acid (BetA). Twenty-five BetA derivatives were synthesized, and evaluated for their antitumor activities against MGC-803, PC3, Bcap-37, A375, and MCF-7 human cancer cell lines by MTT assay. Among the derivatives, most of the derivatives had significant antiproliferative ability (IC50 < 19 μM). Compound 3k, the most active compound, showed IC50 values of 3.6, 5.6, 4.2, 7.8, and 5.2 μM on the five cancer cell lines respectively, and was selected to investigate cell apoptosis by subsequent florescence staining and flow cytometry analysis. The results revealed that compound 3k could induce apoptosis in MGC-803 cell lines, and the apoptosis ratios reached 28.33% after 36 h of treatment at 10 μM. In addition, the study of cancer cell apoptotic signaling pathway indicated that the apoptosis of MGC-803 cells induced by compound 3k could be through the mitochondrial intrinsic pathway.
Keywords: Antitumor; Apoptosis; Betulonic acid derivatives; Mitochondrial pathway; Synthesis. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)