| In vitro: |
| Proceedings of the National Academy of Sciences of the United States of America, 2003,100(11):6646-6651. | | Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7.[Reference: WebLink] | Certain C8-substituted and N7, C8-disubstituted Guanine ribonucleosides comprise a class of small molecules with immunostimulatory activity. In a variety of animal models, these agents stimulate both humoral and cellular immune responses. The antiviral actions of these guanosine analogs have been attributed to their ability to induce type I IFNs. However, the molecular mechanisms by which the guanosine analogs potentiate immune responses are not known.
METHODS AND RESULTS:
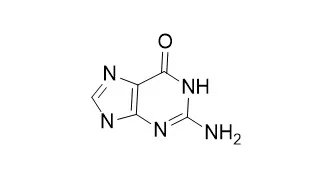
Here, we report that several guanosine analogs activate Toll-like receptor 7 (TLR7). 7-Thia-8-oxoguanosine, 7-deazaguanosine, and related guanosine analogs activated mouse immune cells in a manner analogous to known TLR ligands, inducing cytokine production in mouse splenocytes (IL-6 and IL-12, type I and II IFNs), bone marrow-derived macrophages (IL-6 and IL-12), and in human peripheral blood leukocytes (type I IFNs, tumor necrosis factor alpha and IL-12). The guanosine congeners also up-regulated costimulatory molecules and MHC I/II in dendritic cells. Genetic complementation studies in human embryonic kidney 293 cells confirmed that the guanosine analogs activate cells exclusively via TLR7. The stimulation of TLR7 by the guanosine analogs in human cells appears to require endosomal maturation because inhibition of this process with chloroquine significantly reduced the downstream activation of NF-kappaB. However, TLR8 activation by R-848 and TLR2 activation by [S-[2,3-bis(palmitoyloxy)-(2-RS)-propyl]-N-palmitoyl-R-Cys-S-Ser-Lys4-OH, trihydrochloride)] were not inhibited by chloroquine, whereas TLR9 activation by CpG oligodeoxynucleotides was abolished. In summary, we present evidence that guanosine analogs activate immune cells via TLR7 by a pathway that requires endosomal maturation.
CONCLUSIONS:
Thus, the B cell-stimulating and antiviral activities of the guanosine analogs may be explained by their TLR7-activating capacity. |
|
| In vivo: |
| N Engl J Med, 1997, 337(24):1720-1725. | | The association of atopy with a gain-of-function mutation in the alpha subunit of the interleukin-4 receptor.[Reference: WebLink] | Atopic diseases are very common, and atopy has a strong genetic predisposition.
METHODS AND RESULTS:
Using single-strand conformation polymorphism analysis and DNA sequencing, we searched for mutations in the a subunit of the interleukin-4 receptor that would predispose persons to atopy. We examined the prevalence of the alleles among patients with allergic inflammatory disorders and among 50 prospectively recruited adults. Subjects with atopy were identified on the basis of an elevated serum IgE level (> or = 95 IU per milliliter) or a positive radioimmunosorbent test in response to standard inhalant allergens. The signaling function of mutant interleukin-4 receptor a was examined by flow cytometry, binding assays, and immunoblotting.A novel interleukin-4 receptor alpha allele was identified in which Guanine was substituted for adenine at nucleotide 1902, causing a change from glutamine to arginine at position 576 (R576) in the cytoplasmic domain of the interleukin-4 receptor alpha protein. The R576 allele was common among patients with allergic inflammatory disorders (found in 3 of 3 patients with the hyper-IgE syndrome and 4 of 7 patients with severe atopic dermatitis) and among the 50 prospectively recruited adults (found in 13 of 20 subjects with atopy and 5 of 30 without atopy; P=0.001; relative risk of atopy among those with a mutant allele, 9.3).
CONCLUSIONS:
The R576 allele was associated with higher levels of expression of CD23 by interleukin-4 than the wild-type allele. This enhanced signaling was associated with a change in the binding specificity of the adjacent tyrosine residue at position 575 to signal-transducing molecules.The R576 allele of interleukin-4 receptor alpha is strongly associated with atopy. This mutation may predispose persons to allergic diseases by altering the signaling function of the receptor. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)