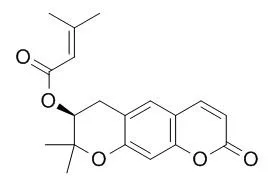
| Description: |
Decursin has antiepileptic, hepatoprotective, anti-cancer, anti-inflammatory, and anti-amnesic activities, it is a novel candidate for inhibition of VEGF-induced angiogenesis. Decursin inhibited the TGF-β1 induced NOX activation and Smad signaling, it inhibited the PKCα, MAPK and NF-κB pathways. Decursin is also a novel inhibitor of NF-kappaB activation in signaling induced by TLR ligands and cytokines. |
| Targets: |
NADPH-oxidase | TGF-β/Smad | AChR | NF-kB | MMP(e.g.TIMP) | p38MAPK | PKC | p53 | P450 (e.g. CYP17) | VEGFR | JNK | LTR | IL Receptor | TNF-α |
| In vitro: |
| Int J Oncol. 2014 May;44(5):1607-13. | | Decursin prevents TPA-induced invasion through suppression of PKCα/p38/NF-κB-dependent MMP-9 expression in MCF-7 human breast carcinoma cells.[Pubmed: 24604087] | Decursin, a coumarin compound, was first isolated from the roots of Angelica gigas almost four decades ago. It was found to exhibit cytotoxicity against various human cancer cells and to possess anti-amnesic activity in vivo through the inhibition of AChE activity. However, the effect of Decursin on breast cancer invasion is unknown. Matrix metalloproteinase-9 (MMP-9) is known to be an important factor for cancer cell invasion.
METHODS AND RESULTS:
Therefore, in this study, we investigated the inhibitory effect of Decursin on 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced MMP-9 expression and cell invasion, as well as the molecular mechanisms involved in MCF-7 cells. Our results showed that Decursin inhibits TPA-induced MMP-9 expression and cell invasion through the suppression of NF-κB. Furthermore, Decursin repressed the TPA-induced phosphorylation of p38 MAPK and inhibited TPA-induced translocation of PKCα from the cytosol to the membrane, but did not affect the translocation of PKCδ.
CONCLUSIONS:
These results indicate that Decursin-mediated inhibition of TPA-induced MMP-9 expression and cell invasion involves the suppression of the PKCα, MAPK and NF-κB pathways in MCF-7 cells. Thus, Decursin may have potential value in restricting breast cancer metastasis. | | Carcinogenesis. 2009 Apr;30(4):655-61. | | Decursin and decursinol angelate inhibit VEGF-induced angiogenesis via suppression of the VEGFR-2-signaling pathway.[Pubmed: 19228635 ] | Inhibition of angiogenesis is an attractive approach for the treatment of angiogenic diseases, such as cancer. Vascular endothelial growth factor (VEGF) is one of the most important activators of angiogenesis and interacts with the high-affinity tyrosine kinase receptors, VEGFR-1 and VEGFR-2.
The pyranocoumarin compounds Decursin and Decursinol angelate isolated from the herb, Angelica gigas, are known to possess potent anti-inflammatory activities. However, little is known about their antiangiogenic activity or their underlying mechanisms.
METHODS AND RESULTS:
Here, we show the antiangiogenic effects of Decursin and Decursinol angelate using in vitro assays and in vivo animal experiments. Decursin and Decursinol angelate inhibited VEGF-induced angiogenic processes in vitro, including proliferation, migration and tube formation of human umbilical vein endothelial cells. Decursin and Decursinol angelate significantly suppressed neovessel formation in chick chorioallantoic membrane and tumor growth in a mouse model. The microvessel density in tumors treated with Decursin for 14 days was significantly decreased compared with a vehicle control group. Decursin and Decursinol angelate inhibited VEGF-induced phosphorylation of VEGFR-2, extracellular signal-regulated kinases and c-Jun N-terminal kinase mitogen-activated protein kinases.
CONCLUSIONS:
Taken together, these results demonstrate that Decursin and Decursinol angelate are novel candidates for inhibition of VEGF-induced angiogenesis. | | Am J Cancer Res . 2019 Sep 1;9(9):2007-2018. | | Decursin inhibits tumor growth, migration, and invasion in gastric cancer by down-regulating CXCR7 expression[Pubmed: 31598401] | | CXC chemokine receptor 7 (CXCR7) is highly expressed in various type of cancers and promotes cancer progression and metastasis. However, the biological role and regulation of CXCR7 in gastric cancer remains unclear, and little is known about compounds that modulate CXCR7. Here, we investigated the role of CXCR7 in gastric tumorigenesis, and the effects of Decursin, which is derived from Angelica gigas Nakai, on CXCR7. Our results showed that CXCR7 significantly promoted growth of gastric cancer cells and increased migration and invasion, which was mediated by the STAT3/c-Myc pathway. We also confirmed that Decursin had an antitumor effect through down-regulating the expression of CXCR7 in gastric cancer. Furthermore, apoptotic cell death was induced through the reduction of anti-apoptotic factors such as Bcl-2 in vitro and in vivo. Our findings show that CXCR7 in gastric cancer promotes cancer progression through the STAT3/c-Myc pathway and that Decursin is a natural compound that may target CXCR7 in gastric cancer treatment. |
|
| In vivo: |
| Life Sci. 2014 Jul 17;108(2):94-103. | | Decursin attenuates hepatic fibrogenesis through interrupting TGF-beta-mediated NAD(P)H oxidase activation and Smad signaling in vivo and in vitro.[Pubmed: 24880074] | We studied that a potent antifibrotic effect of Decursin on in vivo liver damage model and the mechanism in inhibiting which transforming growth factor (TGF)-β1-induced human hepatic stellate cells (HSCs) activation.
METHODS AND RESULTS:
Liver injury was induced in vivo by intraperitoneal injection of carbon tetrachloride (CCl4) with or without Decursin for 4weeks in mice. Human hepatic stellate cell line, an immortalized human HSC line, was used in in vitro assay system. The effects of Decursin on HSC activation were measured by analyzing the expression of α-smooth muscle actin (α-SMA) and collagen I in liver tissue and human HSCs.
Decursin treatment significantly reduced the ratio of liver/body weight, α-SMA activation, and type I collagen overexpression in CCl4 treated mice liver. The elevated serum levels, including ALT, AST, and ALP, were also decreased by Decursin treatment. Treatment of Decursin markedly proved the generation of reactive oxygen species, NAD(P)H oxidase (NOX) protein (1, 2, and 4) upregulation, NOX activity, and superoxide anion production in HSCs by TGF-β1. It also significantly reduced TGF-β1-induced Smad 2/3 phosphorylation, nuclear translocation of Smad 4, and association of Smad 2/3-Smad 4 complex. Consistent with in vitro results, Decursin treatment effectively blocked the levels of NOX protein, and Smad 2/3 phosphorylation in injured mice liver.
CONCLUSIONS:
Decursin blocked CCl4-induced liver fibrosis and inhibited TGF-β1-mediated HSC activation in vitro. These data demonstrated that Decursin exhibited hepatoprotective effects on experimental fibrosis, potentially by inhibiting the TGF-β1 induced NOX activation and Smad signaling. | | Neuroreport. 2014 Nov 12;25(16):1243-9. | | Decursin attenuates kainic acid-induced seizures in mice.[Pubmed: 25171200] | Epilepsy is a neurological disorder with recurrent unprovoked seizures as the main symptom. Of the coumarin derivatives in Angelica gigas, Decursin, a major coumarin component, was reported to exhibit significant protective activity against glutamate-induced neurotoxicity when added to primary cultures of rat cortical cells.
This study served to investigate the effects of Decursin on a kainic acid (KA)-induced status epilepticus model.
METHODS AND RESULTS:
Thirty minutes after intraperitoneal injections of Decursin (20 mg/kg) in male 7-week-old C57BL/6 mice, the animals were treated with KA (30 mg/kg, intraperitoneally) and then examined for behavioral seizure score, electroencephalogram, seizure-related expressed protein levels, neuronal cell loss, neurodegeneration, and astrogliosis. KA injections significantly enhanced neurodegenerative conditions but treatment with Decursin 30 min before KA injection reduced the detrimental effects of KA in mice. The Decursin-treated KA-injected group showed significantly decreased behavioral seizure activity and remarkably attenuated intense and high-frequency seizure discharges in the parietal cortex for 2 h compared with the group treated only with KA. Furthermore, in-vivo results indicated that Decursin strongly inhibits selective neuronal death, astrogliosis, and oxidative stress induced by KA administration.
CONCLUSIONS:
Therefore Decursin is able to attenuate KA-induced seizures and could have potential as an antiepileptic drug. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)