| Structure Identification: |
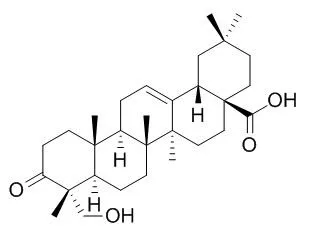
| Chinese Journal of Natural Medicines, 2010,8 (6) :441-448. | | Synthesis and Biological Evaluation of Arjunolic Acid, Bayogenin, Hederagonic Acid and 4-Epi-hederagonic Acid as Glycogen Phosphorylase Inhibitors[Reference: WebLink] | To study glycogen phosphorylase inhibitory activity of natural pentacyclic triterpenes bearing 23-hydroxy or 24-hydroxy.
METHODS AND RESULTS:
Arjunolic acid, bayogenin, Hederagonic acid and 4-epi-Hederagonic acid were synthesized from oleanolic acid as the starting material and biologically evaluated as glycogen phosphorylase inhibitors. Arjunolic acid, bayogenin, Hederagonic acid and 4-epi-Hederagonic acid were successfully semi-synthesized by multiple steps. The synthesis of arjunolic acid was via 11 steps in about 10% overall yield, and bayogenin via 14 steps in about 12% overall yield. Biological evaluation indicated that arjunolic acid, bayogenin, Hederagonic acid and 4-epi-Hederagonic acid showed moderate potency of glycogen phosphorylase inhibition with IC50 of 53-103 μmol/L.
CONCLUSIONS:
Arjunolic acid, bayogenin, Hederagonic acid and 4-epi-Hederagonic acid are gly-cogen phosphorylase inhibitors with moderate potency. Insert of 23-hydroxy or 24-hydroxy to oleanane skeleton has a tendency to be unfavorable to GP inhibitio |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)