| Animal Research: |
| J Nat Med. 2014 Jul;68(3):561-6. | | Acylated phenylethanoid glycosides, echinacoside and acteoside from Cistanche tubulosa, improve glucose tolerance in mice.[Pubmed: 24748124] |
METHODS AND RESULTS:
Acylated phenylethanoid glycosides, echinacoside (1) and acteoside (2), principal constituents in stems of Cistanche tubulosa (Orobanchaceae), inhibited the increase in postprandial blood glucose levels in starch-loaded mice at doses of 250-500 mg/kg p.o. These compounds (1 and 2) also significantly improved glucose tolerance in starch-loaded mice after 2 weeks of continuous administration at doses of 125 and/or 250 mg/kg/day p.o. without producing significant changes in body weight or food intake.
CONCLUSIONS:
In addition, several constituents from C. tubulosa, including 1 (IC50 = 3.1 μM), 2 (1.2 μM), isoacteoside (3, 4.6 μM), 2'-Acetylacteoside (4, 0.071 μM), tubulosides A (5, 8.8 μM) and B (9, 4.0 μM), syringalide A 3-O-α-L-rhamnopyranoside (10, 1.1 μM), campneoside I (13, 0.53 μM), and kankanoside J1 (14, 9.3 μM), demonstrated potent rat lens aldose reductase inhibitory activity. In particular, the potency of compound 4 was similar to that of epalrestat (0.072 μM), a clinical aldose reductase inhibitor. |
|
| Structure Identification: |
| Planta Med. 2001 Aug;67(6):520-2. | | Purification of phenylethanoids from Brandisia hancei and the antiproliferative effects on aortic smooth muscle.[Pubmed: 11509971 ] |
METHODS AND RESULTS:
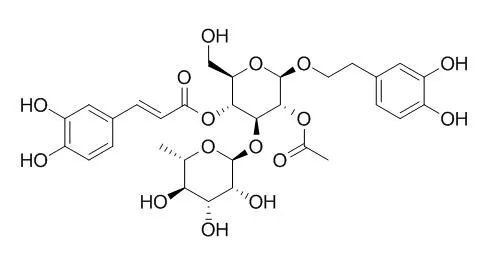
The present study describes the isolation and purification of acteoside, 2'-Acetylacteoside, poliumoside and brandioside, four phenylethanoid glycosides from Brandisia hancei. We examined their effects on the proliferation of cultured A7r5 rat aortic smooth muscle cells. The proliferative response was measured from the [(3)H]-thymidine incorporation into DNA. All four glycosides suppressed the proliferative response in the presence of 2 % or 5 % fetal bovine serum in a concentration-dependent manner. The rank order of effectiveness for inhibition of cell proliferation was: brandioside > or = poliumoside > 2'-Acetylacteoside > or = acteoside. The acetyl group at position 2' of glucose does not seem necessary for the anti-proliferative effects of acteoside and 2'-Acetylacteoside, while the hydroxy groups of the aromatic rings appear to play a role.
CONCLUSIONS:
Inhibition of smooth muscle cell proliferation by phenylethanoids indicates that these compounds may have preventative effects on arteriosclerosis. | | Zhong Yao Cai. 2009 Jul;32(7):1067-9. | | Structure-activity relationships of phenylethanoid glycosides in plants of Cistanche salsa on antioxidative activity.[Pubmed: 19873735] | To study the structure-activity relationships of phenylethanoid glycosides in plants of Cistanche salsa on antioxidative activity.
METHODS AND RESULTS:
By the assay systems of DPPH*, the antioxidant activity of six phenylethanoid glycosides from plants of Cistanche salsa was determined to investigate the relationship between the antioxidant activities and phenylethanoid glycosides's structural characteristics. The antioxidative activity of phenylethanoid glycosides was variant with dose-dependent effect. The sequence of the strength of the antioxidative activity of the six components was shown to be 2'-Acetylacteoside > Acteoside > or = Tubuloside B > or = Isoacteoside > Echinacoside > Cistanoside A.
CONCLUSIONS:
The antioxidative activity of phenylethanoid glycosides is related to the number of phenolic hydroxyl, steric hindrance, 2-acetyl on the middle glucopyranose, and the location of phenolic hydroxyl. Additionally, it may be related to the alpha, beta-unsaturated ketone of phenl-2-propenoyl. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)