| In vitro: |
| Planta Med. 1989 Apr;55(2):166-8. | | Hinokiflavone, a cytotoxic principle from Rhus succedanea and the cytotoxicity of the related biflavonoids.[Pubmed: 2526343] | Hinokiflavone (1) was isolated as the cytotoxic principle from the drupes of Rhus succedanea L.
METHODS AND RESULTS:
A comparison of the cytotoxicity of 1 and other related biflavonoids, including amentoflavone (2), robustaflavone (3), agathisflavone (4), rhusflavone (5), rhusflavanone (6) and its hexaacetate (7), succedaneaflavanone (8) and its hexaacetate (9), cupressuflavone (10), neorhusflavanone (11), volkensiflavone (12) and its hexamethyl ether (13), spicataside (14) and its nonaacetate (15), morelloflavone (16) and its heptaacetate (17) and heptamethyl ether (18), GB-1a (19) and its hexamethyl ether (20) and 7"-O-beta-glucoside (21), and GB-2a (22), indicates that an ether linkage between two units of apigenin as seen in 1 is structurally required for significant cytotoxicity. Compounds 13 and 20 also demonstrated significant cytotoxicity. | | Elife . 2017 Sep 8;6:e27402. | | Characterisation of the biflavonoid hinokiflavone as a pre-mRNA splicing modulator that inhibits SENP[Pubmed: 28884683] | | Abstract
We have identified the plant biflavonoid Hinokiflavone as an inhibitor of splicing in vitro and modulator of alternative splicing in cells. Chemical synthesis confirms Hinokiflavone is the active molecule. Hinokiflavone inhibits splicing in vitro by blocking spliceosome assembly, preventing formation of the B complex. Cells treated with Hinokiflavone show altered subnuclear organization specifically of splicing factors required for A complex formation, which relocalize together with SUMO1 and SUMO2 into enlarged nuclear speckles containing polyadenylated RNA. Hinokiflavone increases protein SUMOylation levels, both in in vitro splicing reactions and in cells. Hinokiflavone also inhibited a purified, E. coli expressed SUMO protease, SENP1, in vitro, indicating the increase in SUMOylated proteins results primarily from inhibition of de-SUMOylation. Using a quantitative proteomics assay we identified many SUMO2 sites whose levels increased in cells following Hinokiflavone treatment, with the major targets including six proteins that are components of the U2 snRNP and required for A complex formation.
Keywords: RNA splicing; SENP1; SUMO; U2 snRNP; biochemistry; Hinokiflavone; none. |
|


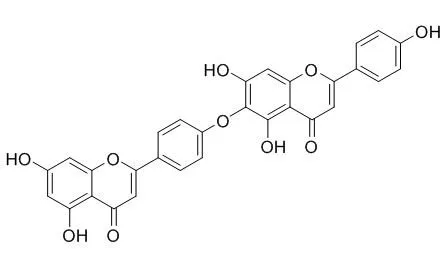



 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)