| Description: |
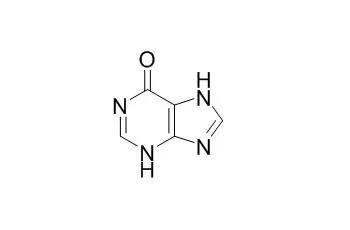
Hypoxanthine, a purine derivative, is a potential free radical generator and could be used as an indicator of hypoxia.Hypoxanthine inhibits mouse oocyte maturation, it plays a role in vivo in maintaining meiotic arrest. |
| In vitro: |
| Dev Biol. 1987 Feb;119(2):313-21. | | The effect of hypoxanthine on mouse oocyte growth and development in vitro: maintenance of meiotic arrest and gonadotropin-induced oocyte maturation.[Pubmed: 3100361] | The concentration of Hypoxanthine in mouse follicular fluid has been estimated to be 2-4 mM, and although this concentration maintains meiotic arrest in fully grown mouse oocytes in vitro, oocyte maturation in vivo is not induced by a decrease in the concentration of this purine in follicular fluid (J. J. Eppig, P. F. Ward-Bailey, and D. L. Coleman, Biol. Reprod. 33, 1041-1049, 1985).
METHODS AND RESULTS:
In the present study, the effect of 2 mM Hypoxanthine on oocyte growth and development in vitro was assessed and the ability of gonadotropins to stimulate oocyte maturation in the continued presence of Hypoxanthine was determined. Oocyte-granulosa cell complexes were isolated from 10- to 11-day-old mice and cultured in the presence or absence of 2 mM Hypoxanthine. Oocytes from 10- to 11-day-old mice are in mid-growth phase and, without further development, are incompetent of undergoing meiotic maturation. During a 12-day culture period the granulosa cell-enclosed oocytes approximately doubled in size and, regardless of the presence or absence of Hypoxanthine, 50-70% developed competence to undergo germinal vesicle breakdown (GVBD). Hypoxanthine promoted the continued association of oocytes with their companion granulosa cells during the 12-day culture period, and therefore had a beneficial effect on oocyte development. Most of the oocytes that acquired GVBD competence in the absence of Hypoxanthine underwent spontaneous GVBD. In contrast, 95% of the GVBD-competent oocytes were maintained in meiotic arrest by Hypoxanthine. Following withdrawal of the Hypoxanthine after the 12-day culture, 75% of the GVBD-competent oocytes underwent GVBD.
CONCLUSIONS:
These results show that Hypoxanthine, and/or its metabolites, maintains meiotic arrest in oocytes that grow and acquire GVBD competence in vitro. Follicle-stimulating hormone (FSH), but not luteinizing hormone or human chorionic gonadotropin, induced oocyte GVBD in the continued presence of Hypoxanthine. FSH stimulated oocyte maturation at a significantly (P less than 0.01) higher frequency than coculture of the granulosa cell-denuded oocytes with granulosa cells in the continued presence of Hypoxanthine. FSH did not induce the maturation of denuded oocytes cocultured with granulosa cells. |
|
| In vivo: |
| Proceedings of the National Academy of Sciences of the United States of America, 1985, 82(2):454-8. | | Hypoxanthine is the principal inhibitor of murine oocyte maturation in a low molecular weight fraction of porcine follicular fluid.[Reference: WebLink] | Studies were carried out to identify and quantify the porcine follicular fluid (PFF) component(s) responsible for inhibition of murine oocyte maturation.
METHODS AND RESULTS:
A low molecular weight fraction of PFF (less than 1000) was prepared by dialysis and used in all experiments. This PFF fraction contained an inhibitor(s) of mouse oocyte maturation that absorbed maximally in the ultraviolet (UV) range at 250-260 nm. When the PFF fraction was charcoal-extracted, significant loss of absorbance at 250, 260, and 280 nm resulted, which corresponded to loss of inhibitory activity. Four major components of PFF were separated by ion-exchange chromatography and characterized according to their UV spectral characteristics and inhibitory activity. When individual fractions demonstrating identical spectra were pooled and analyzed by high-performance liquid chromatography, the first pooled fraction (A) was found to be impure, but adenine comprised 80% of the UV-absorbing material. Fractions B, C, and D were characterized as pure uracil, Hypoxanthine, and 7-methylinosine, respectively. The concentrations of these compounds in PFF were estimated to be 0.06 mM adenine, 0.44 mM uracil, 1.41 mM Hypoxanthine, and 0.19 mM 7-methylinosine. Comparison of the potencies of commercial preparations of these compounds established that Hypoxanthine is the major inhibitory component of the low molecular weight PFF fraction. Moreover, a commercial preparation of Hypoxanthine mimicked the action of PFF on mouse oocyte maturation in that it produced a transient inhibition of oocyte maturation that was significantly potentiated by follicle-stimulating hormone and dibutyryl cyclic adenosine monophosphate. When the inhibitory efficacies of purine and pyrimidine bases and nucleosides were compared, their relative potencies in decreasing order were purine bases greater than purine nucleosides greater than pyrimidine bases = pyrimidine nucleosides.
CONCLUSIONS:
We conclude that Hypoxanthine is the predominant low molecular weight component of PFF that inhibits mouse oocyte maturation but that other purines/pyrimidines may also play a role in vivo in maintaining meiotic arrest. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)