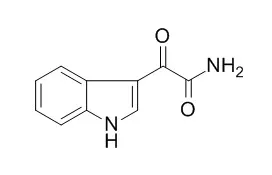
| Structure Identification: |
| J Med Chem. 2015 Dec 10;58(23):9309-33. | | An Orally Bioavailable, Indole-3-glyoxylamide Based Series of Tubulin Polymerization Inhibitors Showing Tumor Growth Inhibition in a Mouse Xenograft Model of Head and Neck Cancer.[Pubmed: 26580420 ] |
METHODS AND RESULTS:
A number of Indole-3-glyoxylamides have previously been reported as tubulin polymerization inhibitors, although none has yet been successfully developed clinically. We report here a new series of related compounds, modified according to a strategy of reducing aromatic ring count and introducing a greater degree of saturation, which retain potent tubulin polymerization activity but with a distinct SAR from previously documented libraries. A subset of active compounds from the reported series is shown to interact with tubulin at the colchicine binding site, disrupt the cellular microtubule network, and exert a cytotoxic effect against multiple cancer cell lines.
CONCLUSIONS:
Two compounds demonstrated significant tumor growth inhibition in a mouse xenograft model of head and neck cancer, a type of the disease which often proves resistant to chemotherapy, supporting further development of the current series as potential new therapeutics. | | Eur J Med Chem. 2011 Sep;46(9):4125-32. | | Discovery of 6-substituted indole-3-glyoxylamides as lead antiprion agents with enhanced cell line activity, improved microsomal stability and low toxicity.[Pubmed: 21726921 ] |
A series of highly potent Indole-3-glyoxylamide based antiprion agents was previously characterized, focusing on optimization of structure-activity relationship (SAR) at positions 1-3 of the indole system.
METHODS AND RESULTS:
New libraries interrogating the SAR at indole C-4 to C-7 now demonstrate that introducing electron-withdrawing substituents at C-6 may improve biological activity by up to an order of magnitude, and additionally confer higher metabolic stability. For the present screening libraries, both the degree of potency and trends in SAR were consistent across two cell line models of prion disease, and the large majority of compounds showed no evidence of toxic effects in zebrafish.
CONCLUSIONS:
The foregoing observations thus make the Indole-3-glyoxylamides an attractive lead series for continuing development as potential therapeutic agents against prion disease. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)