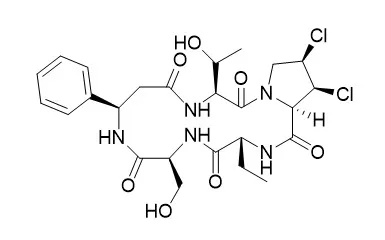
| Structure Identification: |
| J Sep Sci. 2015 Feb;38(4):571-5. | | Simultaneous separation and determination of phenolic acids, pentapeptides, and triterpenoid saponins in the root of Aster tataricus by high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry.[Pubmed: 25491750 ] |
METHODS AND RESULTS:
We established a qualitative method to analyze the main chemical compositions of the root of Aster tataricus. Most of the peaks were separated on a C(18) column packed with 5.0 μm particles, and 28 compounds were identified, including 11 chlorogenic acids, ten astins/asterinins, and seven astersaponins, four of which were reported for the first time from A. tataricus. Furthermore, we developed a reliable method for the simultaneous quantification of 3-caffeoylquinic acid, 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, Astin A, astin B, astin C, astersaponin A, and astersaponin C, and the qualified separations were achieved only on a C18 column packed with 2.7 μm particles.
CONCLUSIONS:
The method was used to measure the concentrations of eight components in samples from two major producing areas in China, and the average contents in samples from Bozhou (Anhui) were higher than those in samples from Anguo (Hebei). | | Chem Pharm Bull (Tokyo). 1996 May;44(5):1026-32. | | Cyclic peptides from higher plants. XXVIII. Antitumor activity and hepatic microsomal biotransformation of cyclic pentapeptides, astins, from Aster tataricus.[Pubmed: 8689717 ] |
METHODS AND RESULTS:
Antitumor activities on Sarcoma 180A of a series of cyclic pentapeptides, astins, isolated from the roots of Aster tataricus, were examined.
CONCLUSIONS:
The activities on various congeners of the dichlorinated proline residues prepared by chemical conversion and a hepatic microsomal biotransformation in rats suggested that 1,2-cis dichlorinated proline residues of Astin A, astin B and astin C play an important role in the antitumor activity of astins. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)