Hydroxylation of steroids at one of the side chain terminal methyl groups, commonly linked to C-26, represents an important regulatory step established in many phyla. Discrimination between the two sites, C-26 and C-27, requires knowing the stereochemistry of the products. 26-Hydroxylation of the insect steroid hormone 20-hydroxyecdysone by a microsomal cytochrome P450 was previously found to be responsible for hormonal resistance in a Chironomus cell line mainly producing the (25S)-epimer of 20,26-dihydroxyecdysone.
METHODS AND RESULTS:
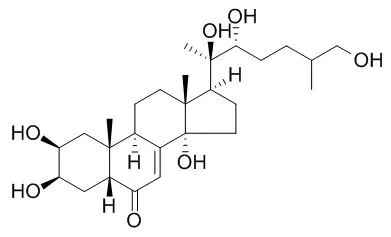
Here, we studied the 25-desoxy analog of 20-hydroxyecdysone, ponasterone A, to elucidate the stereochemistry of the expected 26-hydroxy product, Inokosterone, which occurs as C-25 epimers in nature. We identified the predominant metabolite as the C-25 R epimer of Inokosterone on comparison by RP-HPLC with the (25R)- and (25S)-epimers the stereochemistry of which was confirmed by X-ray crystallography. (25R)-Inokosterone was further oxidized to the 26-aldehyde identified by mass spectroscopy, borohydride reduction and metabolic transformation to 26-carboxylic acid. The (25S)-epimers of Inokosterone and its aldehyde were minor products. With 20-hydroxyecdysone as substrate, we newly identified the (25R)-epimer of 20,26-dihydroxyecdysone as a minor product.
CONCLUSIONS:
In conclusion, the present stereochemical studies revealed high regioselectivity of the Chironomus enzyme to hydroxylate both steroids at the same methyl group, denoted C-27. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)