| Kinase Assay: |
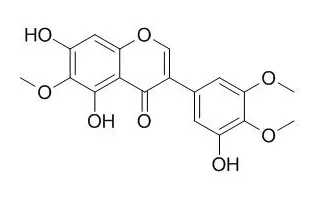
| Life Sci. 2006 Apr 11;78(20):2336-42. | | Inhibitory effects of Irigenin from the rhizomes of Belamcanda chinensis on nitric oxide and prostaglandin E(2) production in murine macrophage RAW 264.7 cells.[Pubmed: 16307761 ] |
METHODS AND RESULTS:
In the present study, we investigated antiinflammatory effects of six flavonoids isolated from the rhizomes of Belamcanda chinensis (Iridaceae) in RAW 264.7 macrophages. The results indicated that Irigenin concentration dependently inhibited lipopolysaccharide (LPS)-induced nitric oxide (NO) and prostaglandin (PG) E(2) production. Furthermore, this compound inhibited the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 proteins and mRNAs without an appreciable cytotoxic effect. Treatment of the transfectant RAW 264.7 cells with Irigenin reduced the level of nuclear factor-kappaB (NF-kappaB) activity, also effectively lowered NF-kappaB binding measured by electrophoretic mobility shift assay (EMSA), which was associated with decreased p65 protein levels in the nucleus. On the basis of the above data, we suggest that the effect of Irigenin in decreasing LPS-induced NO and PGE(2) synthesis is due to diminish the mRNA and protein expression of iNOS and COX-2, respectively, also may be due to under the suppression of NF-kappaB activation.
CONCLUSIONS:
Therefore, Irigenin isolated from the rhizomes of Belamcanda chinensis could be offered as a leading compound for anti-inflammation. |
|
| Cell Research: |
| Planta Med. 2003 Jan;69(1):15-20. | | Cancer chemopreventive in vitro activities of isoflavones isolated from Iris germanica.[Pubmed: 12567273 ] |
METHODS AND RESULTS:
Six known isoflavones were isolated from the rhizomes of Iris germanica, and were established by UV, MS and NMR techniques as irisolidone (1), irisolidone 7-O-alpha-D-glucoside (1a), Irigenin (2), irilone (3), iriflogenin (4), and iriskashmirianin (5). These compounds were examined for their cancer chemopreventive potential. They were shown to be potent inhibitors of cytochrome P450 1A activity with IC 50 values in the range 0.25-4.9 microM. The isoflavones 2, 3 and 5 displayed moderate activity as inducers of NAD(P)H:quinone reductase (QR) in cultured mouse Hepa 1c1c7 cells, with CD values (concentration required to double the specific activity of QR) of 3.5-16.7 microM, whereas weak activity was observed with compounds 4 and 5 in the radical (DPPH) scavenging bioassay (IC 50 values 89.6 and 120.3 microM, respectively).
CONCLUSIONS:
With respect to anti-tumor promoting potential based on anti-inflammatory mechanisms, none of the compounds demonstrated significant activity in the concentration range tested. |
|
| Structure Identification: |
| J Sep Sci. 2017 Jun;40(12):2565-2574. | | Ionic-liquid-based ultrasound-assisted extraction of isoflavones from Belamcanda chinensis and subsequent screening and isolation of potential α-glucosidase inhibitors by ultrafiltration and semipreparative high-performance liquid chromatography.[Pubmed: 28444982 ] | The separation of a compound of interest from its structurally similar homologues to produce high-purity natural products is a challenging problem.
METHODS AND RESULTS:
This work proposes a novel method for the separation of iristectorigenin A from its structurally similar homologues by ionic-liquid-based ultrasound-assisted extraction and the subsequent screening and isolation of potential α-glucosidase inhibitors via ultrafiltration and semipreparative high-performance liquid chromatography. Ionic-liquid-based ultrasound-assisted extraction was successfully applied to the extraction of tectorigenin, iristectorigenin A, Irigenin, and irisflorentin from Belamcanda chinensis. The optimum conditions for the efficient extraction of isoflavones were determined as 1.0 M 1-ethyl-3-methylimidazolium tetrafluoroborate with extraction time of 30 min and a solvent to solid ratio of 30 mL/g. Ultrafiltration with liquid chromatography and mass spectrometry was applied to screen and identify α-glucosidase inhibitors from B. chinensis, followed by the application of semipreparative high-performance liquid chromatography to separate and isolate the active constituents. Four major compounds including tectorigenin, iristectorigenin A, Irigenin, and irisflorentin were screened and identified as α-glucosidase inhibitors, and then the four active compounds abovementioned were subsequently isolated by semipreparative high-performance liquid chromatography (99.89, 88.97, 99.79, and 99.97% purity, respectively).
CONCLUSIONS:
The results demonstrate that ionic liquid extraction can be successfully applied to the extraction of isoflavones from B. chinensis. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)