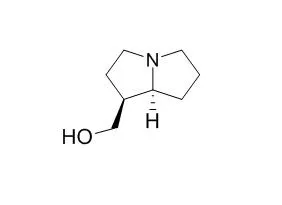
| Structure Identification: |
| Tetrahedron,2014,70(2):204–11. | | Asymmetric syntheses of (−)-isoretronecanol and (−)-trachelantamidine[Reference: WebLink] | Short and concise total asymmetric syntheses of (−)-Isoretronecanol and (−)-trachelantamidine are reported.
METHODS AND RESULTS:
Oxidative cleavage of tert-butyl (S,S,S,Z)-7-[N-benzyl-N-(α-methylbenzyl)amino]cyclohept-3-ene-1-carboxylate, followed by hydrogenolysis promoted in situ cyclisation/reduction, which provided rapid access to the bicyclic core within (−)-Isoretronecanol. Analogous treatment of the C(1)-epimer gave (−)-trachelantamidine.
CONCLUSIONS:
Overall, the syntheses of (−)-Isoretronecanol and (−)-trachelantamidine were completed in eight and seven steps and 20 and 9.5% yield, respectively, from commercially available starting materials. | | Tetrahedron Letters,2005,46(15);2691-3. | | The stereoselective addition of titanium(IV) enolates of 1,3-oxazolidin-2-one and 1,3-thiazolidine-2-thione to cyclic N-acyliminium ion. The total synthesis of (+)-isoretronecanol[Reference: WebLink] |
METHODS AND RESULTS:
(+)-Isoretronecanol (1) has been prepared in four steps and 36% overall yield via the diastereoselective addition of the titanium(IV) enolate derived from N-4-chlorobutyryl-1,3-thiazolidine-2-thione (3) to N-Boc-2-methoxypyrrolidine (5), which afforded 2-substituted pyrrolidine 7 in 84% yield (8:1 diastereoisomeric ratio), followed by reductive recovery of the chiral auxiliary and cyclization. | | Tetrahedron: Asymmetry,2011,22(6):662–668. | | A common approach to pyrrolizidine and indolizidine alkaloids; formal synthesis of (−)-isoretronecanol, (−)-trachelanthamidine and an approach to the synthesis of (−)-5-epitashiromine and (−)-tashiromine[Reference: WebLink] |
METHODS AND RESULTS:
A common and short stereoselective route is described for the formal synthesis of pyrrolizidine alkaloids, (−)-Isoretronecanol and (−)-trachelanthamidine. An approach to the synthesis of indolizidine alkaloids (−)-5-epitashiromine and (−)-tashiromine utilizing ring closing metathesis is also described starting from commercially available and inexpensive l-proline. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)