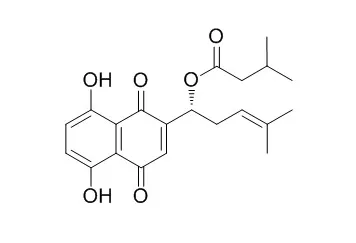
Leukemia remains life-threatening despite remarkable advances in chemotherapy. The poor prognosis and drug resistance are challenging treatment. Novel drugs are urgently needed. Shikonin, a natural naphthoquinone, has been previously shown by us to be particularly effective towards various leukemia cell lines compared to solid tumors. However, the underlying mechanisms are still poorly understood.
METHODS AND RESULTS:
Here, we investigated shikonin and 14 derivatives on U937 leukemia cells. Four derivatives (isobutyrylshikonin, 2-methylbutyrylshikonin, Isovalerylshikonin and β,β-dimethylacrylshikonin) were more active than shikonin. AnnexinV-PI analysis revealed that shikonins induced apoptosis. Cell cycle G1/S check point regulation and the transcription factor c-MYC, which plays a vital role in cell cycle regulation and proliferation, were identified as the most commonly down-regulated mechanisms upon treatment with shikonins in mRNA microarray hybridizations. Western blotting and DNA-binding assays confirmed the inhibition of c-MYC expression and transcriptional activity by shikonins. Reduction of c-MYC expression was closely associated with deregulated ERK, JNK MAPK and AKT activity, indicating their involvement in shikonin-triggered c-MYC inactivation. Molecular docking studies revealed that shikonin and its derivatives bind to the same DNA-binding domain of c-MYC as the known c-MYC inhibitors 10058-F4 and 10074-G5. This finding indicates that shikonins bind to c-MYC. The effect of shikonin on U937 cells was confirmed in other leukemia cell lines (Jurkat, Molt4, CCRF-CEM, and multidrug-resistant CEM/ADR5000), where shikonin also inhibited c-MYC expression and influenced phosphorylation of AKT, ERK1/2, and SAPK/JNK.
CONCLUSIONS:
In summary, inhibition of c-MYC and related pathways represents a novel mechanism of shikonin and its derivatives to explain their anti-leukemic activity. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)