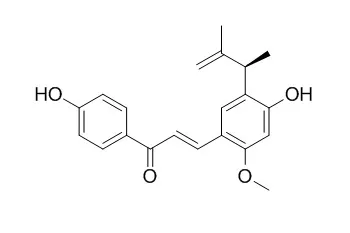
| Description: |
Licochalcone E is a potential LXRβ agonist, which has chemopreventive, cytotoxic, anti-inflammatory, antimicrobial, antidiabetic effects; it increases the levels of PPARγ expression, at least in part, via the stimulation of Akt signals and functions as a PPARγ partial agonist. Licochalcone E may be used for the treatment of hepatotoxicity, and primarily exhibits its protective role through a PPARγ/NF-κB-mediated pathway. Licochalcone E is also a potential activator of the Nrf2/ARE-dependent pathway and is therapeutically relevant not only to oxidative-stress-related neurodegeneration but also inflammatory responses of microglial cells both in vitro and in vivo. |
| In vitro: |
| Zhongguo Zhong Yao Za Zhi. 2016 Aug;41(16):3065-3071. | | Discovery of potential LXRβ agonists from Chinese herbs using molecular simulation methods.[Pubmed: 28920350] |
Liver X receptor β (LXRβ) has been a new target in the treatment of hyperlipemia, which was related to the cholesterol homeostasis.
METHODS AND RESULTS:
In this study, the quantitative pharmacophores were constructed by 3D-QSAR pharmacophore (Hypogen) method based on the LXRβ agonists. The optimal pharmacophore model containing one hydrogen bond acceptor, two hydrophobics and one ring aromatic was obtained based on five assessment indictors, including the correlation between predicted value and experimental value of the compounds in training set (correlation), Δcost of the models (Δcost), hit rate of active compounds (HRA), identification of effectiveness index (IEI) and comprehensive evaluation index (CAI). And the values of the five assessment indicators were 0.95, 128.65, 84.44%, 2.58 and 2.18 respectively. The best model as a query to screen the traditional Chinese medicine database (TCMD), a list of 309 compounds was obtained andwere then refined using Libdock program. Finally, based on the screening rules of the Libdock score of initial compound and the key interactions between initial compound and receptor, four compounds, demethoxycurcumin, isolicoflavonol, Licochalcone E and silydianin, were selected as potential LXRβ agonists.
CONCLUSIONS:
The molecular simulation methods were high-efficiency and time-saving to obtainthe potential LXRβ agonists, which could provide assistance for further researchingnovel anti-hyperlipidemia drugs. | | J Microbiol Biotechnol. 2012 Jun;22(6):800-5. | | Antimicrobial activity of licochalcone E against Staphylococcus aureus and its impact on the production of staphylococcal alpha-toxin.[Pubmed: 22573157] | Licochalcone E was firstly isolated from licorice root in 2005, which belongs to the retrochalcone family. Studies on the biological activities of Licochalcone E were in the initial stage.
METHODS AND RESULTS:
In the study, we demonstrated that Licochalcone E has potent antimicrobial property against Staphylococcus aureus. Furthermore, via hemolysis, Western blot, and real-time RT-PCR assays, we have shown that subinhibitory concentrations of Licochalcone E dosedependently reduces the production of alpha-toxin in both methicillin-sensitive S. aureus (MSSA) and methicillinresistant S. aureus (MRSA).
CONCLUSIONS:
The data suggest that Licochalcone E may deserve further investigation as a potential therapeutic against S. aureus infections, or the structure of Licochalcone E may be used as a basis for chemical synthesis of novel anti-S. aureus compounds. | | J Nutr Biochem. 2012 Jul;23(7):759-67. | | Licochalcone E has an antidiabetic effect.[Pubmed: 21840191] | Licochalcone E (lico E) is a retrochalcone isolated from the root of Glycyrrhiza inflata. Retrochalcone compounds evidence a variety of pharmacological profiles, including anticancer, antiparasitic, antibacterial, antioxidative and superoxide-scavenging properties.
METHODS AND RESULTS:
In this study, we evaluated the biological effects of lico E on adipocyte differentiation in vitro and obesity-related diabetes in vivo. We employed 3T3-L1 preadipocyte and C3H10T1/2 stem cells for in vitro adipocyte differentiation study and diet-induced diabetic mice for in vivo study. The presence of lico E during adipogenesis induced adipocyte differentiation to a significant degree, particularly at the early induction stage. Licochalcone E evidenced weak, but significant, peroxisome proliferator-activated receptor gamma (PPARγ) ligand-binding activity. Two weeks of lico E treatment lowered blood glucose levels and serum triglyceride levels in the diabetic mice. Additionally, treatment with lico E resulted in marked reductions in adipocyte size and increases in the mRNA expression levels of PPARγ in white adipose tissue (WAT). Licochalcone E was also shown to significantly stimulate Akt signaling in epididymal WAT.
CONCLUSIONS:
In conclusion, lico E increases the levels of PPARγ expression, at least in part, via the stimulation of Akt signals and functions as a PPARγ partial agonist, and this increased PPARγ expression enhances adipocyte differentiation and increases the population of small adipocytes, resulting in improvements in hyperglycemia and hyperlipidemia under diabetic conditions. | | Int Immunopharmacol. 2010 Sep;10(9):1119-26. | | Licochalcone E reduces chronic allergic contact dermatitis and inhibits IL-12p40 production through down-regulation of NF-kappa B.[Pubmed: 20601178 ] | Licochalcone, a constituent of licorice, has antitumor, antimicrobial, and anti-inflammatory effects. Recently, Licochalcone E was isolated from the roots of Glycyrrhiza inflata and its biological functions are not fully examined.
METHODS AND RESULTS:
In this study, we investigated its ability to modulate production of IL-12p40, a common subunit of IL-12 and IL-23. Licochalcone E dose-dependently inhibited IL-12p40 production from lipopolysaccharide-stimulated RAW264.7 macrophage cells. The repressive effect was mapped to a region in the IL-12 gene promoter containing a binding site for NF-kappaB. Furthermore, Licochalcone E decreased binding to the NF-kappaB site in RAW264.7 macrophage cells. Using a chronic allergic contact dermatitis model induced by repeated application of oxazolone, we showed that Licochalcone E inhibited the increased IL-12p40 expression and ear thickness induced by oxazolone.
CONCLUSIONS:
Taken together, Licochalcone E inhibits IL-12p40 production and has therapeutic potential to reduce skin inflammation. |
|
| In vivo: |
| Cancer Prev Res (Phila). 2013 Jun;6(6):603-13. | | Licochalcone E present in licorice suppresses lung metastasis in the 4T1 mammary orthotopic cancer model.[Pubmed: 23625311] | We investigated whether Licochalcone E (LicE), a phenolic constituent of licorice, inhibits mammary tumor growth and metastasis using animal and cell culture models.
METHODS AND RESULTS:
4T1 mammary carcinoma cells were injected into the mammary fat pads of syngeneic BALB/c mice. Starting 7 days after the injection, the mice received LicE (7 or 14 mg/kg body weight/day) via oral gavage for 25 days. LicE suppressed solid tumor growth and lung metastasis, but did not exhibit kidney or liver toxicity. In tumor tissues, LicE treatment induced a reduction in the expression of Ki67, cyclins, and cyclin-dependent kinases and stimulated apoptosis with increased expression of Bax and cleaved caspase-3 but decreased expression of Bcl-2. In addition, LicE decreased expression of CD31, vascular endothelial growth factor (VEGF)-A and C, VEGF-receptor 2, lymphatic vessel endothelial receptor-1, CD45, cyclooxygenase-2, inducible nitric oxide synthase, and hypoxia inducible factor-1α in tumor tissues. In lung tissues, LicE reduced the levels of proinflammatory cytokines and angiogenesis/metastasis-related proteins. In mammary cancer cell cultures, LicE (5-20 μmol/L) dose dependently inhibited cell migration and invasion. LicE inhibited secretion of matrix metalloproteinase-9, urokinase-type plasminogen activator and VEGF-A, and stimulated secretion of tissue inhibitor of metalloproteinase-2 in MDA-MB-231 cells. In addition, LicE inhibited tube formation of vascular endothelial cells.
CONCLUSIONS:
We show that LicE administration suppressed tumor growth and lung metastasis in the mouse model in conjunction with LicE inhibition of cell migration, invasion, and tube formation in vitro. Reduced tumor growth and metastasis in LicE-treated mice may be, at least in part, attributed to reduced inflammation and tumor angiogenesis. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)