| In vitro: |
| Comb Chem High Throughput Screen. 2013 Feb;16(2):160-6. | | Flavonoids and linderone from Lindera oxyphylla and their bioactivities.[Pubmed: 23173924] |
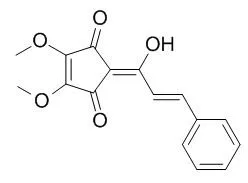
A new Linderone A, namely 2-cinnamoyl-3-hydroxy-4, 5-dimethoxycyclopenta-2, 4-dienone (5), together with three known flavonoids (1-3) and one Linderone (4), were isolated from the bark of Lindera oxyphylla.
METHODS AND RESULTS:
Extensive spectroscopic analysis including 1D and 2D-NMR spectra determined their sturctures. In addition, the antioxidant activity of all the compounds has been determined using 2, 2-diphenyl-1-picrylhydrazyl radical scavenging (DPPH), ferric reducing antioxidant power (FRAP) and ferrous ion chelating (FIC) methods. Compound 3 showed excellent DPPH scavenging activity with IC50% value of 8.5 ± 0.004% (μg/mL) which is comparable with vitamin C. This compound, also showed an absorbance value of 1.00 ± 0.06% through FRAP test when compared with Butyl Hydroxy Aniline (BHA). However, FIC showed low activity for all the isolated compounds (chelating activity less than 50%) in comparison with ethylene diamine tetra acetic acid (EDTA). Anticancer activity for all compounds has also been measured on A375 human melanoma, HT-29 colon adenocarcinoma, MCF-7 human breast adenocarcinoma cells, WRL-68 normal hepatic cells, A549 non-small cell lung cancer cells and PC-3 prostate adenocarcinoma cell line.
CONCLUSIONS:
Compound 1 showed A549=65.03%, PC-3=30.12%, MCF-7=47.67, compound 2 showed PC-3=90.13%, compound 3 showed MCF-7=79.57 and for compound 5 MCF-7 is 96.33. | | Org Lett. 2010 May 21;12(10):2354-7. | | Bi-linderone, a highly modified methyl-linderone dimer from Lindera aggregata with activity toward improvement of insulin sensitivity in vitro.[Pubmed: 20420414] | Bi-Linderone (1) was isolated as racemate from the traditional Chinese medicinal plant Lindera aggregata.
METHODS AND RESULTS:
The structure elucidation of bi-Linderone was reported on the basis of extensive analysis of NMR spectra and crystal X-ray diffraction. Bi-Linderone has an unprecedented spirocyclopentenedione-containing carbon skeleton and showed significant activity against glucosamine-induced insulin resistance in HepG2 cells at a concentration of 1 microg/mL. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)