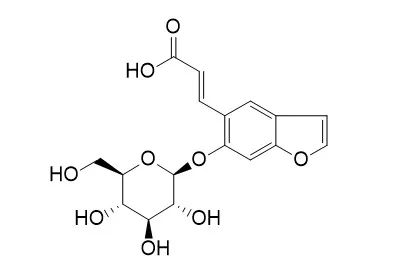
| In vitro: |
| Saudi Pharm J . 2014 Nov;22(5):460-71 | | Phytochemical and pharmacological study of Ficus palmata growing in Saudi Arabia[Pubmed: 25473335] | | Phytochemical study of the aerial parts of Ficus palmata utilizing liquid-liquid fractionation and different chromatographic techniques resulted in the isolation of a new isomer of psoralenoside namely, trans-Psoralenoside (5) in addition to, one triterpene: germanicol acetate (1), two furanocoumarins: psoralene (2), bergapten (3), one aromatic acid vanillic acid (4) and the flavone glycoside rutin (6). Structures of the isolated compounds were established through physical, 1D- and 2D-NMR and MS data. The total extract and fractions of the plant were examined in vivo for its possible effects as hepatoprotective, nephroprotective, antiulcer and anticoagulant activities in comparison with standard drugs. Hepatoprotective activity was assessed via serum biochemical parameters including aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyl transpeptidase (GGT), alkaline phosphatase (ALP) and total bilirubin. Tissue parameters such as non-protein sulfhydryl groups (NP-SH), malonaldehyde (MDA) and total protein (TP) were also measured. In addition to tissue parameters, nephroprotective effect was evaluated by measuring the serum levels of sodium, potassium, creatinine and urea. Histopathological study for both liver and kidney cells was also conducted. Antiulcer activity was explored by observing stomach lesions after treatment with ethanol. Whole blood clotting time (CT) was taken as a measure for the anticoagulant activity of the extract. Antioxidant activity of the total extract and fractions of the plant was measured using 2,2-diphenyl-1-picrylhydrazyl (DPPH) method and ascorbic acid as standard. | | Saudi Pharm J. 2014 Nov;22(5):460-471. | | Phytochemical and pharmacological study of Ficus palmata growing in Saudi Arabia[Pubmed: 25473335] | | Phytochemical study of the aerial parts of Ficus palmata utilizing liquid-liquid fractionation and different chromatographic techniques resulted in the isolation of a new isomer of psoralenoside namely, trans-Psoralenoside (5) in addition to, one triterpene: germanicol acetate (1), two furanocoumarins: psoralene (2), bergapten (3), one aromatic acid vanillic acid (4) and the flavone glycoside rutin (6). Structures of the isolated compounds were established through physical, 1D- and 2D-NMR and MS data. The total extract and fractions of the plant were examined in vivo for its possible effects as hepatoprotective, nephroprotective, antiulcer and anticoagulant activities in comparison with standard drugs. Hepatoprotective activity was assessed via serum biochemical parameters including aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyl transpeptidase (GGT), alkaline phosphatase (ALP) and total bilirubin. Tissue parameters such as non-protein sulfhydryl groups (NP-SH), malonaldehyde (MDA) and total protein (TP) were also measured. In addition to tissue parameters, nephroprotective effect was evaluated by measuring the serum levels of sodium, potassium, creatinine and urea. Histopathological study for both liver and kidney cells was also conducted. Antiulcer activity was explored by observing stomach lesions after treatment with ethanol. Whole blood clotting time (CT) was taken as a measure for the anticoagulant activity of the extract. Antioxidant activity of the total extract and fractions of the plant was measured using 2,2-diphenyl-1-picrylhydrazyl (DPPH) method and ascorbic acid as standard. |
|
| In vivo: |
| Saudi Pharm J. 2014 Nov;22(5):460-471. | | Phytochemical and pharmacological study of Ficus palmata growing in Saudi Arabia[Pubmed: 25473335] | | Phytochemical study of the aerial parts of Ficus palmata utilizing liquid-liquid fractionation and different chromatographic techniques resulted in the isolation of a new isomer of psoralenoside namely, trans-Psoralenoside (5) in addition to, one triterpene: germanicol acetate (1), two furanocoumarins: psoralene (2), bergapten (3), one aromatic acid vanillic acid (4) and the flavone glycoside rutin (6). Structures of the isolated compounds were established through physical, 1D- and 2D-NMR and MS data. The total extract and fractions of the plant were examined in vivo for its possible effects as hepatoprotective, nephroprotective, antiulcer and anticoagulant activities in comparison with standard drugs. Hepatoprotective activity was assessed via serum biochemical parameters including aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyl transpeptidase (GGT), alkaline phosphatase (ALP) and total bilirubin. Tissue parameters such as non-protein sulfhydryl groups (NP-SH), malonaldehyde (MDA) and total protein (TP) were also measured. In addition to tissue parameters, nephroprotective effect was evaluated by measuring the serum levels of sodium, potassium, creatinine and urea. Histopathological study for both liver and kidney cells was also conducted. Antiulcer activity was explored by observing stomach lesions after treatment with ethanol. Whole blood clotting time (CT) was taken as a measure for the anticoagulant activity of the extract. Antioxidant activity of the total extract and fractions of the plant was measured using 2,2-diphenyl-1-picrylhydrazyl (DPPH) method and ascorbic acid as standard. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)