| In vitro: |
| Planta Medica, 2011 , 77 (12):PG42. | | New biphenyl derivatives and anti-inflammatory constituents from the stem bark of Magnolia officinalis[Reference: WebLink] |
The stem bark of Magnolia officinalis Rehd. et Wils. (Magnoliaceae) has been used as a traditional medicine for the treatment of gastrointestinal disorders, bronchitis, and emphysema, in China, Taiwan, Japan, and Korea [1]. Chemical studies have revealed a variety of neo-lignans and alkaloids as constituents of this plant. Many of these compounds exhibit central depressant effect, muscle relaxation, and antigastric ulcer, antibacterial, antiallergic, vasorelaxant, and neurotrophic activities.
METHODS AND RESULTS:
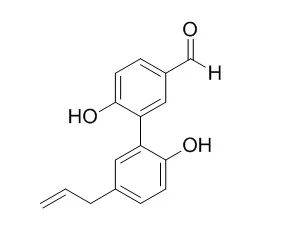
Investigation on EtOAc-soluble fraction of the stem bark of M. officinalis has led to the isolation of three new biphenyls, 5-allyl-5'-(1-hydroxyallyloxy)biphenyl-2,2-diol (1), 5,5'-diallyl-2'-(allyloxy)biphenyl-2-ol (2), and 5,5'-diallyl-2'-(3-methylbut-2-enyloxy)biphenyl-2-ol (3), together with 12 known compounds, including four neolignans, magnolol (4), honokiol (5), (-)-monoterpenylmagnolol (6), and randainal (7), two norlignans, Magnaldehyde D (8) and randaiol (9), and six steroids, β-sitostenone (10), stigmasta-4,22-dien-3-one (11), β-sitosterol (12), stigmasterol (13), 3β-hydroxystigmast-5-en-7-one (14), and 3β-hydroxystigmasta-5,22-dien-7-one (15).
CONCLUSIONS:
The structure of new compounds (1-3) were determined through spectroscopic and MS analyses. Among the isolates, magnolol (4) and honokiol (5) exhibited potent inhibition against fMLP-induced superoxide production with IC50 values of 4.42±0.24 and 0.68±0.20μg/mL, respectively. In addition, magnolol (4) inhibited fMLP/CB-induced elastase release with an IC50 values of 1.45±0.20μg/mL. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)