| Structure Identification: |
| Planta Medica, 2012, 78(11):1204-1204. | | New cromones from Cnidium monnieri fruits inhibit adipocyte differentiation in 3T3-L1 cells.[Reference: WebLink] |
METHODS AND RESULTS:
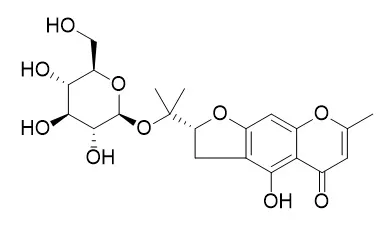
Seven new chromone glycosides, monnieriside A (3),monnieriside B (10), monnieriside C (12) monnieriside D (13), monnieriside E (15), monnieriside F (16), Monnieriside G (17) and one new phenolic compound, methylpicraquassioside B (23) were isolated from the n-BuOH soluble fraction of Cnidium monnieri fruits (Umbelliferae), together with fourteen known compounds, undulatoside A (1), cnidimol C (2), saikochromoside A (4), cnidimoside A (5), cnidimoside B (6), 2-methyl-5-hydroxy-6-(2-butenyl-3-hydroxymethyl)-7-(β-D-glucopyranosyloxy)-4H-1-benzopyran-4-one (7), cnidimol D (8), hydroxycnidimoside A (9), umtatin (11), 6′-hydroxylangelicain (14), cnidioside A (18), cnidioside B (19), picraquassioside A (20), methylpicraquassioside A (21) and picraquassioside B (22). The structures of isolated compounds were determined on the basis of spectroscopic analysis including 1D, 2D NMR and HRESI-MS.
CONCLUSIONS:
Among the compounds isolated, compounds 5, 6, 9 and 10 significantly inhibited adipocyte differentiation as measured by fat accumulation in 3T3-L1 cells using Oil Red O staining. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)