| In vitro: |
| Fitoterapia. 2014 Jan;92:100-4. | | Chemical constituents from Aphanamixis grandifolia.[Pubmed: 24188860] |
METHODS AND RESULTS:
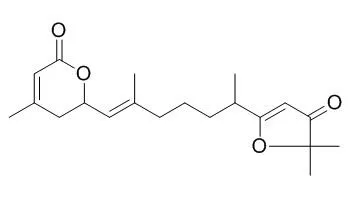
Four new terpenoids, Nemoralisins D-G (1-4), were isolated from the leaves and stems of Aphanamixis grandifolia, along with two known diterpenoids, Nemoralisin C and Nemoralisin. Among them, compound 1 is the first example of norsesquiterpenoid with δ-lactone moiety, and Nemoralisins E-G (2-4), are a class of acyclic diterpenoids, which are structurally related Nemoralisin C and Nemoralisin. These structures were established on the basis of spectroscopic methods and the absolute configuration of 1 was determined by comparison of quantum chemical TDDFT calculated and experimental ECD spectra.
CONCLUSIONS:
Nemoralisins D-G (1-4) were tested for their cytotoxicities on HL-60, SMMC-7721, A-549, MCF-7, and SW480 human tumor cell lines (IC50>40 μM), as well as the antimicrobial activities on Staphylococcus aureus, Pseudomonas aeruginosa, MRSA92(#) and MRSA98(#) (MIC>50 μg/mL). | | Chem Pharm Bull (Tokyo). 2014;62(5):494-8. | | Aphanamixins A-F, acyclic diterpenoids from the stem bark of Aphanamixis polystachya.[Pubmed: 24789934] |
METHODS AND RESULTS:
Six new acyclic diterpenoids named Aphanamixins A-F (1-6), together with two known compounds of Nemoralisin and Nemoralisin C, were isolated from the stem bark of Aphanamixis polystachya (WALL) J. N. BARKER. Their structures were established through a comprehensive analysis of NMR spectroscopic data and high resolution mass spectrometric data. The absolute configurations of carbon stereocenters were determined by means of auxiliary chiral α-methoxy-α-(trifluoromethyl)phenylacetic acid (MTPA) derivatives and circular dichroism (CD), respectively.
CONCLUSIONS:
All the new isolates were tested for their antiproliferative activity against HepG2, AGS, MCF-7, and A-549 cancer cell lines and they exhibited weak cytotoxicities (IC50>10 µM). Moreover, we highlighted that the six new diterpenoids characterized by acyclic skeleton was rarely seen in nature. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)