| Kinase Assay: |
| Biochim Biophys Acta. 2014 Dec;1840(12):3311-9. | | Tiliroside, a dietary glycosidic flavonoid, inhibits TRAF-6/NF-κB/p38-mediated neuroinflammation in activated BV2 microglia.[Pubmed: 25152356] | Tiliroside is a dietary glycosidic flavonoid which has shown in vivo anti-inflammatory activity. This study is aimed at evaluating the effect of Tiliroside on neuroinflammation in BV2 microglia, and to identify its molecular targets of anti-neuroinflammatory action.
METHODS AND RESULTS:
BV2 cells were stimulated with LPS+IFNγ in the presence or absence of Tiliroside. TNFα, IL-6, nitrite and PGE2 production was determined with ELISA, Griess assay and enzyme immunoassay, respectively. iNOS, COX-2, phospho-p65, phospho-IκBα, phospho-IKKα, phospho-p38, phospho-MK2, phosopho-MKK3/6 and TRAF-6 were determined by western blot analysis. NF-κB activity was also investigated using a reporter gene assay in HEK293 cells. LPS-induced microglia ROS production was tested using the DCFDA method, while HO-1 and Nrf2 activation was determined with western blot. RESULTS: Tiliroside significantly suppressed TNFα, IL-6, nitrite and PGE2 production, as well as iNOS and COX-2 protein expression from LPS+IFNγ-activated BV2 microglia. Further mechanistic studies showed that Tiliroside inhibited neuroinflammation by targeting important steps in the NF-κB and p38 signalling in LPS+IFNγ-activated BV2 cells. This compound also inhibited LPS-induced TRAF-6 protein expression in BV2 cells. Antioxidant activity of Tiliroside in BV2 cells was demonstrated through attenuation of LPS+IFNγ-induced ROS production and activation of HO-1/Nrf2 antioxidant system.
CONCLUSIONS:
Tiliroside inhibits neuroinflammation in BV2 microglia through a mechanism involving TRAF-6-mediated activation of NF-κB and p38 MAPK signalling pathways. These activities are possibly due, in part, to the antioxidant property of this compound. Tiliroside is a potential novel natural compound for inhibiting neuroinflammation in neurodegenerative disorders. | | Biol Pharm Bull. 1998 Oct;21(10):1077-8. | | Anti-complement activity of tiliroside from the flower buds of Magnolia fargesii.[Pubmed: 9821813] | As part of the search for anticomplementary active components from natural products, the anticomplementary properties of methanolic extracts from the flower buds of Magnoliafargesii have been investigated.
METHODS AND RESULTS:
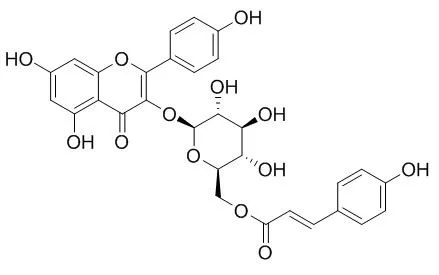
Bioassay-guided chromatographic separation of the active constituents led to the isolation of compound 1, whose structure was identified by spectroscopic methods to be kaempferol 3-O-beta-D-(6"-O-coumaroyl)glucopyranoside (Tiliroside). Tiliroside showed very potent anti-complement activity (IC50=5.4 x 10(-5) M) on the classical pathway of the complement system, even higher than rosmarinic acid, which is a well-known inhibitor against the complement system. On the other hand, the hydrolysates of Tiliroside, kaempferol, astragalin and p-coumaric acid showed very weak activity on this system. |
|
| Animal Research: |
| J Nutr Biochem. 2012 Jul;23(7):768-76. | | Tiliroside, a glycosidic flavonoid, ameliorates obesity-induced metabolic disorders via activation of adiponectin signaling followed by enhancement of fatty acid oxidation in liver and skeletal muscle in obese-diabetic mice.[Pubmed: 21889885] | Tiliroside contained in several dietary plants, such as rose hips, strawberry and raspberry, is a glycosidic flavonoid and possesses anti-inflammatory, antioxidant, anticarcinogenic and hepatoprotective activities. Recently, it has been reported that the administration of Tiliroside significantly inhibited body weight gain and visceral fat accumulation in normal mice.
METHODS AND RESULTS:
In this study, we evaluated the effects of Tiliroside on obesity-induced metabolic disorders in obese-diabetic KK-A(y) mice. In KK-A(y) mice, the administration of Tiliroside (100 mg/kg body weight/day) for 21 days failed to suppress body weight gain and visceral fat accumulation. Although Tiliroside did not affect oxygen consumption, respiratory exchange ratio was significantly decreased in mice treated with Tiliroside. In the analysis of metabolic characteristics, it was shown that plasma insulin, free fatty acid and triglyceride levels were decreased, and plasma adiponectin levels were increased in mice administered Tiliroside. The messenger RNA expression levels of hepatic adiponectin receptor (AdipoR)-1 and AdipoR2 and skeletal muscular AdipoR1 were up-regulated by Tiliroside treatment. Furthermore, it was indicated that Tiliroside treatment activated AMP-activated protein kinase in both the liver and skeletal muscle and peroxisome proliferator-activated receptor α in the liver. Finally, Tiliroside inhibited obesity-induced hepatic and muscular triglyceride accumulation.
CONCLUSIONS:
These findings suggest that Tiliroside enhances fatty acid oxidation via the enhancement adiponectin signaling associated with the activation of both AMP-activated protein kinase and peroxisome proliferator-activated receptor α and ameliorates obesity-induced metabolic disorders, such as hyperinsulinemia and hyperlipidemia, although it does not suppress body weight gain and visceral fat accumulation in obese-diabetic model mice. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)