| Structure Identification: |
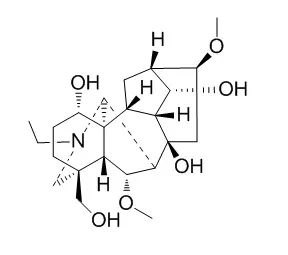
| Planta Med. 2011 Mar;77(4):368-73. | | Diterpene alkaloids from Aconitum anthora and assessment of the hERG-inhibiting ability of Aconitum alkaloids.[Pubmed: 20862641 ] | A new norditerpene alkaloid, 10-hydroxy-8- O-methyltalatizamine (1), was isolated from the whole plant of ACONITUM ANTHORA L. besides the known isotalatizidine (2) and hetisinone (3).
METHODS AND RESULTS:
The structures were determined by means of HR-ESI-MS, 1D and 2D NMR spectroscopy, including 1H-1H COSY, NOESY, HSQC and HMBC experiments, resulting in complete 1H and 13C chemical shift assignments for 1- 3, and revision of some earlier 13C-NMR data.
The effects of the isolated compounds, together with twenty-one other ACONITUM alkaloids with different skeletal types and substitution patterns, on hERG channels were studied by the whole-cell patch clamp technique, using the QPatch-16 automated patch clamp system.
CONCLUSIONS:
At 10 μM, aconitine, 14-benzoylaconine 8- O-palmitate, songoramine, gigactonine and Neolinine demonstrated significant hERG K+ channel inhibition; all other compounds exerted only low (6-21%) inhibitory activity. | | Chinese Traditional & Herbal Drugs,1996,27(1):5-8. | | Studies on the Diterpenoid Alkaloids of Yiyulongwutou (Aconitum pseudostapfianum)[Reference: WebLink] |
METHODS AND RESULTS:
From the roots of Aconitum pseudostapfianum W. T. Warig,ten diterpenoid alkaloids were isolated and identified as 14-benzoyl-8-O-methylaconine (Ⅰ ),aconitine(Ⅱ),deoxyaconitine(Ⅲ),penduline (Ⅳ),yunaconitine (Ⅴ), Neolinine (Ⅵ), 15α-hydroxyneoline (Ⅶ), neoline (Ⅷ), talatisamine (Ⅸ)and aconosine (Ⅹ). I has not been previously found in nature. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)