| Kinase Assay: |
| Phytochemistry. 2012 Apr;76:162-71. | | Inhibition of saturated very-long-chain fatty acid biosynthesis by mefluidide and perfluidone, selective inhibitors of 3-ketoacyl-CoA synthases.[Pubmed: 22284369] | The trifluoromethanesulphonanilides mefluidide and perfluidone are used in agriculture as plant growth regulators and herbicides. Despite the fact that mefluidide and perfluidone have been investigated experimentally for decades, their mode of action is still unknown.
METHODS AND RESULTS:
In this study, we used a cascade approach of different methods to clarify the mode of action and target site of mefluidide and perfluidone. Physiological profiling using an array of biotests and metabolic profiling in treated plants of Lemna paucicostata suggested a common mode of action in very-long-chain fatty acid (VLCFA) synthesis similar to the known 3-ketoacyl-CoA synthase (KCS) inhibitor metazachlor. Detailed analysis of fatty acid composition in Lemna plants showed a decrease of saturated VLCFAs after treatment with mefluidide and perfluidone. To study compound effects on enzyme level, recombinant KCSs from Arabidopsis thaliana were expressed in Saccharomyces cerevisiae. Enzyme activities of seven KCS proteins from 17 tested were characterized by their fatty acid substrate and product spectrum. For the KCS CER6, the VLCFA product spectrum in vivo, which consists of tetracosanoic acid, hexacosanoic acid and Octacosanoic Acid, is reported here for the first time. Similar to metazachlor, mefluidide and perfluidone were able to inhibit KCS1, CER6 and CER60 enzyme activities in vivo. FAE1 and KCS2 were inhibited by mefluidide only slightly, whereas metazachlor and perfluidone were strong inhibitors of these enzymes with IC(50) values in μM range.
CONCLUSIONS:
This suggests that KCS enzymes in VLCFA synthesis are the primary herbicide target of mefluidide and perfluidone. |
|
| Structure Identification: |
| Zhong Yao Cai. 2013 May;36(5):739-43. | | Chemical constituents from the aerial part of Echinacea purpurea[Pubmed: 24218964] | To study the chemical constituents of the aerial part of Echinacea purpurea.
METHODS AND RESULTS:
The compounds were separated and purified by repeatedly silica gel, ODS, D101 macroporous resin, MCI, Sephadex LH-20 column chromatography and recrystallization. Their structures were elucidated on the basis of physiochemical properties and spectral analysis.
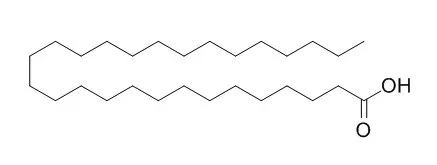
Sixteen compounds were isolated and identified as (2S)-1-O-octacosanoyl glycerol (1), (5R,6S)-6-hydroxy-6-((E)-3-hydroxybut-1-enyl)-1,1, 5-trimethylcyclohexanone (2), (3S, 6E, 10R)-3, 10, 11-trihydroxy-3, 7, 11-trimethyl-dodeca-1, 6-diene (3), negunfurol (4), schensianol A (5), ent-4 (15) -eudesmene-1beta, 6alpha-diol (6), (E) -5-hydroxy-N-isobutylpentadec-2-enamide (7), syringaresinol (8), quercetin (9), ethyl laurate (10), ethyl caffeate (11), ferulic acid (12), alpha-spinasterol (13), stigmasterol (14), beta-daucosterol (15), Octacosanoic Acid (16).
CONCLUSIONS:
Compound 1 - 5 are isolated from the Asteraceae for the first time, compound 6 ,7, 9, 10, 12 are isolated from genus of Echinacea for the first time, compound 15, 16 are isolated from this plant for the first time. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)