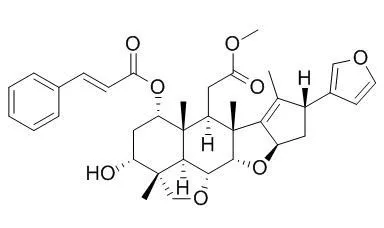
| In vitro: |
| Chem Biodivers. 2014 Aug;11(8):1121-39. | | Cytotoxic and nitric oxide production-inhibitory activities of limonoids and other compounds from the leaves and bark of Melia azedarach.[Pubmed: 25146759 ] | Nine limonoids, 1-9, one apocarotenoid, 11, one alkaloid, 12, and one steroid, 13, from the leaf extract; and one triterpenoid, 10, five steroids, 14-18, and two flavonoids, 19 and 20, from the bark extract of Melia azedarach L. (Chinaberry tree; Meliaceae) were isolated.
METHODS AND RESULTS:
Among these compounds, three compounds, 4-6, were new, and their structures were established as 3-deacetyl-28-oxosalannolactone, 3-deacetyl-28-oxosalanninolide, and 3-deacetyl-17-defurano-17,28-dioxosalannin, respectively, on the basis of extensive spectroscopic analyses and comparison with literature data. All of the isolated compounds were evaluated for their cytotoxic activities against leukemia (HL60), lung (A549), stomach (AZ521), and breast (SK-BR-3) cancer cell lines. 3-Deacetyl-4'-demethyl-28-oxosalannin (3) against HL60 and AZ521 cells, and methyl kulonate (10) against HL60 cells exhibited potent cytotoxicities with IC50 values in the range of 2.8-5.8 μM. In addition, upon evaluation of compounds 1-13 against production of nitric oxide (NO) in mouse macrophage RAW 264.7 cells induced by lipopolysaccharide (LPS), seven, i.e., trichilinin B (1), 4, Ohchinin (7), 23-hydroxyOhchininolide (8), 21-hydroxyisoOhchininolide (9), 10, and methyl indole 3-carboxylate (12), inhibited production of NO with IC50 values in the range of 4.6-87.3 μM with no, or almost no, toxicity to the cells (IC50 93.2-100 μM).
CONCLUSIONS:
Western blot analysis revealed that compound 7 reduced the expression levels of the inducible NO synthase (iNOS) and COX-2 proteins in a concentration-dependent manner.
Furthermore, compounds 5, 6, 13, and 18-20 exhibited potent inhibitory effects (IC50 299-381 molar ratio/32 pmol TPA) against Epstein-Barr virus early antigen (EBV-EA) activation induced by 12-O-tetradecanoylphorbol-13-acetate (TPA) in Raji cell line. | | Z Naturforsch C. 1993 May-Jun;48(5-6):495-9. | | Azadirachtin inhibits proliferation of Sf 9 cells in monolayer culture.[Pubmed: 8363710] | Azadirachtin A in ppm quantity inhibits proliferation and monolayer formation of Spodoptera frugiperda (Sf9) insect cells in monolayer culture.
METHODS AND RESULTS:
The incubated cells demonstrate reduced rates of protein synthesis which finally leads to cell death. This growth inhibiting activity is compared with other botanicals such as Ohchinin, salannin and volkensin. The high and specific activity of azadirachtin A on insect cells is discussed in comparison with its effect on a mammalian cell line, based on 2 D PAGE analysis of the total protein contents. | | Chem Biodivers. 2016 Oct;13(10):1410-1421. | | Melanogenesis-Inhibitory Activities of Isomeric C-seco Limonoids and Deesterified Limonoids.[Pubmed: 27450797] |
METHODS AND RESULTS:
Treatment of eight C-seco limonoids including six of salannin-type, 1 - 6, and two of nimbin-type, 7 and 8, with a combination of BF3 · Et2 O and iodide ion yielded the isomeric C-seco derivatives, i.e., six isosalannins, 1a - 6a, and two isonimbins, 7a and 8a, respectively. Ohchinin (1) was further subjected to LiAlH4 reduction which yielded a deesterified trihydroxy limonoid, nimbidinol (9). In addition, ten limonoids including seven of azadirone-type, 10 - 16, and three of gedunin-type, 17 - 19, all of which possess no ester functionality in the molecule, were obtained from the neutral fraction of Azadirachta indica seed extract after alkaline hydrolysis. Among the above, twelve compounds, i.e., 1a - 4a, 6a, 9, 13 - 16, 18, and 19, were new compounds, and their structures were elucidated on the basis of extensive spectroscopic analysis and comparison with literature data.
CONCLUSIONS:
Upon evaluation of all these limonoids for their inhibitory activities against melanogenesis in B16 melanoma cells induced with α-melanocyte-stimulating hormone (α-MSH), five structurally modified limonoids, 3-deacetyl-28-oxosalannin (6a), 9, 17-epi-17-hydroxynimbocinol (14), 17-epi-17-hydroxy-15-methoxynimbocinol (15), and 7-deacetyl-17-epinimolicinol (18), in addition to a natural limonoid, 1, exhibited potent inhibitory activities with 26 - 66% reduction of melanin content at 100 μm concentration with almost no or low toxicity to the B16 melanoma cells (70 - 99% cell viability at 100 μm). |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)