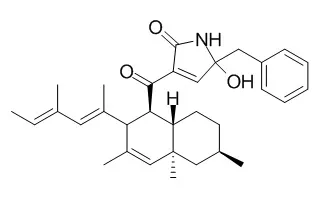
| Structure Identification: |
| Tetrahedron Letters,2013,54(6):506–511. | | First total synthesis of oteromycin utilizing one-pot four-step cascade reaction strategy[Reference: WebLink] | The first total synthesis of Oteromycin was investigated.
METHODS AND RESULTS:
Our previously reported convergent strategy for the synthesis of α-acyl-γ-hydroxy-γ-lactams was first applied for the total synthesis, however, the final deprotection of the methoxyaminal moiety could not be achieved since an unexpected intramolecular Diels–Alder (IMDA) reaction occurred. Therefore, a novel one-pot four-step cascade reaction starting from α-selenolactam was investigated.
CONCLUSIONS:
The efficient synthetic strategy was successfully developed to afford the desired Oteromycin, and its complete structure elucidation including the stereochemistry at C24 position was also accomplished. | | Proceedings of the Symposium on Progress in Organic Reactions and Syntheses. 2009. | | Synthetic Study of HIV Integrase Inhibitor Oteromycin[Reference: WebLink] | Oteromycin is a HIV integrase inhibitor isolated from fungi MF5810 and MF5811, and has attracted a lot of attention for it's constructive features, decalin skeleton and alpha,beta-unsaturated-alpha-acyl-gamma-hydroxylactam moiety.
METHODS AND RESULTS:
In the initial stage of the total synthesis, the novel synthetic method of alpha,beta-unsaturated-alpha-acyl-gamma-hydroxylactam moiety was developed utilizing the catalytic acid-mediated dehydrogenation of alpha-acyl-gamma-hydroxylactam by DDQ. We succeeded in establishing the novel synthetic method, lanched synthesis of the decalin skeleton of Oteromycin. As a key step for the construction of the decalin skeleton, an intramolecular Diels-Alder (IMDA) reaction was adopted.
CONCLUSIONS:
The synthesis of the decalin equipped with all stereogenic centers has been achieved, starting from commercially available (+)-citronellal. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)