| Animal Research: |
| J Ethnopharmacol . 2016 May 13;183:128-135. | | Anti-inflammatory and antinociceptive activities of Croton urucurana Baillon bark[Pubmed: 26944237] | | Ethnopharmacological relevance: Croton urucurana (Euphorbiaceae) is popularly used in Brazil to treat inflammatory processes, pain, and gastric ulcers.
Aim of study: To evaluate the anti-inflammatory and antinociceptive properties of the methanol extract from the bark of C. urucurana (MECu) in mice and identify its chemical constituents.
Materials and methods: The extract was characterized by UHPLC-DAD-ESI-Q-TOF-MS/MS. Extract doses of 25, 100, and 400mg/kg were employed in the biological assays. Evaluation of anti-inflammatory activity was based on paw edema and leukocyte recruitment into the peritoneal cavity of mice, both induced by carrageenan. Abdominal writhing caused by acetic acid and duration of formalin-induced paw-licking were the models employed to evaluate antinociceptive activity.
Results: Ten compounds were identified in the extract: (+)-gallocatechin (1), procyanidin B3 (2), (+)-catechin (3), (-)-epicatechin (4), Tembetarine (5), magnoflorine (6), taspine (7), methyl-3-oxo-12-epi-barbascoate (8), methyl-12-epi-barbascoate (9), and hardwickiic acid (10). This is the first report of compounds 2, 4, 6, 7, and 10 in C. urucurana and compound 5 in the genus Croton. In addition to inhibiting paw edema and leukocyte recruitment (particularly of polymorphonuclear cells) into the peritoneal cavity of mice, MECu reduced the number of abdominal writhings induced by acetic acid and the duration of formalin-induced paw licking.
Conclusions: The methanol extract of C. urucurana bark exhibited anti-inflammatory and antinociceptive properties, corroborating its use in folk medicine. These effects may be related to the presence of diterpenes, alkaloids, and flavonoids. | | Fitoterapia . 2001 Jun;72(5):538-543. | | Antifeedant constituents from Fagara macrophylla[Pubmed: 11429249] | | Analysis of the polar fractions of an EtOH extract obtained from the bark of the African medicinal plant Fagara macrophylla led to the isolation and identification of the alkaloids oblongine (6), Tembetarine (7) and magnoflorine (8) and the flavonoid hesperidin (9). These compounds, together with other metabolites (1--5) previously isolated from F. macrophylla, were tested for antifeedant activity in a binary-choice bioassay. The acridone alkaloid xanthoxoline (4) was found to have a potent antifeedant activity against larvae of both Spodoptera frugiperda and S. littoralis. 1-Hydroxy-3-methoxy-N-methyl-acridone (2), arborinine (3), Tembetarine (7) and magnoflorine (8) were antifeedant against S. frugiperda. |
|
| Structure Identification: |
| Zhongguo Zhong Yao Za Zhi . 2011 Apr;36(8):1024-1027. | | [Study on chemical constituents from branches and leaves of Polyalthia nemoralis][Pubmed: 21809577] | | Objective: To investigate the chemical constituents of the branches and leaves of Polyalthia nemoralis.
Method: The compounds were isolated and purified by silica gel, macroporous adsorption resin and Sephadex LH-20 column chromatographic methods. Their chemical structures were elucidated on the basis of physicochemical properties and spectral data.
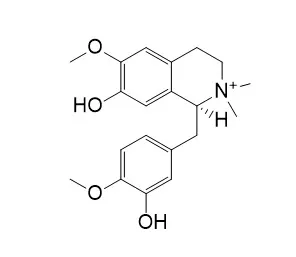
Result: Fourteen compounds were isolated and identified as syringic acid (1), 3-methoxy-4-hydroxycinnamic acid (2), vanillic acid (3), 4-hydroxybenzoic acid (4), mauritianin (5), (+)-xylopinidine (6), (+)-oblongine(7), (+)-Tembetarine (8), eythritol (9), D-mannitol (10), ethyl-beta-D-glucopyranoside (11), (+)-magnoflorine (12), stepharanine (13), (2S, 4R)-4-hydroxy-2-piperidine-carboxylic acid (14), respectively.
Conclusion: All the compounds were isolated from the genus Polyalthia for the first time; compounds 6 and 13 showed inhibitation activities against multi tumor cell lines. | | Phytochemistry . 2004 Apr;65(7):939-944. | | Quaternary isoquinoline alkaloids from Xylopia parviflora[Pubmed: 15081299] | | From the quaternary alkaloidal fraction of the bark and the root of Xylopia parviflora (Annonaceae), four isoquinoline alkaloids, xylopinidine, dehydrocoreximine, N, N-dimethylanomurine and N-methylphoebine were isolated along with the known compounds, pycnarrhine, lotusine, 6,7-dimethoxy-2-methyl-isoquinolinium salt, 1,2-dehydroreticuline, (-)-phellodendrine, (+)-Tembetarine, (-)-litcubine, (+)-magnoflorine, tetradehydroreticuline, (-)-oblongine, (+)-menisperine, (+)-N-methylcorydine, stepharanine, (+)-xanthoplanine, dehydrodiscretine, jatrorrhizine and palmatine. 3,4-Dihydro-6,7-dimethoxy-2-methyl-isoquinolinium and N-methylpurpuerine were isolated as natural products for the first time. Their structures were determined on the basis of spectroscopic evidence. | | Fitoterapia . 2001 Jun;72(5):538-543. | | Antifeedant constituents from Fagara macrophylla[Pubmed: 11429249] | | Analysis of the polar fractions of an EtOH extract obtained from the bark of the African medicinal plant Fagara macrophylla led to the isolation and identification of the alkaloids oblongine (6), Tembetarine (7) and magnoflorine (8) and the flavonoid hesperidin (9). These compounds, together with other metabolites (1--5) previously isolated from F. macrophylla, were tested for antifeedant activity in a binary-choice bioassay. The acridone alkaloid xanthoxoline (4) was found to have a potent antifeedant activity against larvae of both Spodoptera frugiperda and S. littoralis. 1-Hydroxy-3-methoxy-N-methyl-acridone (2), arborinine (3), Tembetarine (7) and magnoflorine (8) were antifeedant against S. frugiperda. | | Molecules . 2013 Jul 3;18(7):7739-7750. | | Studies on the alkaloids of the bark of Magnolia officinalis: isolation and on-line analysis by HPLC-ESI-MS(n)[Pubmed: 23823874] | | The bark of Magnolia officinalis is a well-known Traditional Chinese Medicine. In the present study, two new alkaloids, named (S)-4-keto-magnoflorine (6) and (R)-3,4-dehydromagnocurarine (11), together with seven known alkaloids: (S)-magnoflorine (5), trans/cis N-feruloylputrescine (7/8), (R)-magnocurarine (10), (S)-Tembetarine (12), (R)-oblongine (14), and (R)-asimilobine (17) were isolated and their structures elucidated by spectroscopic methods, including 1D, 2D NMR, and HRESI-MS. The absolute configurations of the isoquinoline alkaloids 5, 6, 10-12, 14, and 17 were determined by CD. In vitro inhibitory activities against aldose reductase, lipase, α-glucosidase, DPP-IV and three cancer cell lines (A549, Bel-7402, and HCT-8) were evaluated for all isolated compounds. However, all compounds showed weak activities in all tests at the same concentration as the positive control drugs. An HPLC-ESI-MS(n) method has been established for screening of alkaloids in the bark of M. officinalis. A total of 23 alkaloids were identified or tentatively characterized; including 13 aporphines, eight benzylisoquinolines and two amides. Plausible fragmentation pathways of the representative compounds 6, 7/8, 11, and 17 were proposed in the present study. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)