| In vitro: |
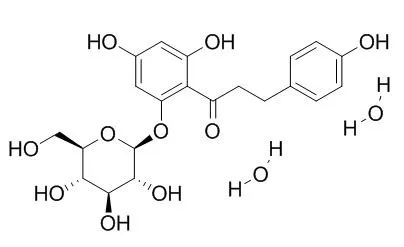
| Biochemical Pharmacology Volume 35, Issue 13, 1 July 1986, Pages 2221–2227 | | Crystal structure of phlorizin and the iodothyronine deiodinase inhibitory activity of phloretin analogues[Reference: WebLink] | Phloretin, a 7,8-dihydrochalcone of plant origin, and the high molecular weight (<15,000) polyphloretinphosphate (PPP) polymers are potent inhibitors of iodothyronine monodeiodinase activity from rat liver microsomal preparations, whereas phlorizin, the 2′-O-glucoside of phloretin, is inactive.
METHODS AND RESULTS:
The polymers, differing in degree of phosphorylation-dependent polymerization, exhibited a concentration-dependent, and ultimately complete, inhibition of deiodinase activity with an IC50 between 0.2 and 0.5 μg PPP/ml. Phloretin inhibition, on the other hand, was cofactor (DTE) competetive, with a Ki = 0.75 μM. 2′,4′,6′,3,4-Pentahydroxychalcone, which has a substitution pattern in the A-ring identical to that of phloretin, was the only active inhibitor (IC50= 8 μM) among several derivatives tested. The phloretin biodegradation products, phloretic acid and phloroglucinol, and its biosynthetic precursors, monomeric cinnamic acid and cinnamic acid derivatives, were inactive in concentrations up to 100 μM. The X-ray crystal structure analysis of Phlorizin dihydrate showed that the molecule is planar and fully extended, similar to the conformation observed in chalcone structures that are characterized by an α, β-unsaturated bond between phenol rings.
CONCLUSIONS:
Comparison of the planar phlorizin crystal structure with a skewed or antiskewed thyroid hormone conformation revealed that the β-D-glucose moiety does not share any of the thyroid hormone's conformational space, and that the best structural homology is found with the antiskewed conformation of 3′,5′,3-triiodothyronine, the natural deiodinase substrate that also inhibits further deiodination.
| | Korean Journal of Plant Resources Vol.23 No.6, 2010.12, 487-497 | | Inhibitory Effect of the Phenolic Compounds from Apples Against Oxidative Damage and Inflammation[Reference: WebLink] | ROS have been associated with pathogenic processes including carcinogenesis through direct effect on DNA and play an important role in the pathogenesis of inflammation. Because of many types of phenolic acid derivatives and flavonoids, apples have been one of the human diet since ancient times and are one of the most commonly consumed fruits in worldwide.
METHODS AND RESULTS:
In this study, catechin, chlorogenic acid and Phlorizin dihydrate were purified and identified by HPLC and GC/MS. The contents of catechin, chlorogenic acid and Phlorizin dihydrate were 1.01 ㎎, 7.01 ㎎ and 3.67 ㎎/ ㎏ wet weight, respectively. Catechin and Phlorizin dihydrate were found to significantly inhibit oxidative DNA damage, while chlorogenic did not affect. Also, catechin inhibits NO and PGE₂ production via suppressing iNOS and COX-2 expression. However, chlorogenic acid and Phlorizin dihydrate did not affect.
CONCLUSIONS:
Our results show that catechin may be the most active phenolic compound in anti-oxidative damage and anti-inflammatory effect. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)