| In vitro: |
| Allelopathy Journal, 2009, 23(2):437-444. | | Effects of applied phthalic acid and phloroglucionol dihydrate on the root oxidative damage in tomato seedlings.[Reference: WebLink] |
METHODS AND RESULTS:
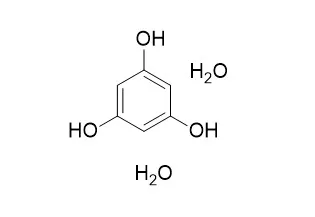
We examined the effects of main autotoxic substances (phthalic acid and phloroglucionl dihydrate, separated in our previous study), on root oxidative damage of tomato seedlings. Changes in superoxide dismutase (SOD, EC 1.15.1.1), peroxidase (POD, EC 1.11.1.7), catalase (CAT, EC 1.11.1.6) and malondialdehyde (MDA) and their activities in roots were measured. Potted tomato seedlings were cultured in perlite and treated with phthalic acid (PA) and Phloroglucinol dihydrate (PD) as exogenous autotoxins at 1 mM, 5 mM and 10 mM concentrations. The application of both PD and PA, and especially PD increased the MDA contents. The activities of SOD, CAT and POD depended on autotoxins (PA or PD), their time of action and concentration. The enzyme activities increased with application of PA on 5th day and decreased on 10th day except at 10 mM PA. On 20th day, the activities of all enzymes decreased except SOD at 1 mM. Similar trend of enzyme changes was presented in the treatments of PD, except POD activity that kept growing on the 10 th day.
CONCLUSIONS:
Results indicated the adverse effects of exogenous PA and PD on enzymes of antioxidant defence system, resulting in lipid peroxidation in roots of tomato seedlings. | | Plos One, 2018, 13(1):e0190509. | | Drug repurposing: In-vitro anti-glycation properties of 18 common drugs.[Reference: WebLink] | Drug repositioning or repurposing, i.e. identifying new indications for existing drugs, has gained increasing attention in the recent years. This approach enables the scientists to discover “new targets” for known drugs in a cost and time efficient manner. Glycation, the non-enzymatic reaction of sugars with proteins or nucleic acids to form early glycation (Amadori or fructosamine) products, is a key molecular basis of diabetic complications. Inhibiting the process of non-enzymatic protein glycation is one of the key strategies to prevent glycation-mediated diabetic complications.
METHODS AND RESULTS:
The present study focuses on the anti-glycation activity of 18 drugs, commonly used for the treatment of gastrointestinal, central nervous system, inflammatory diseases, bacterial infections, and gout. This study was carried out by using two in-vitro protein anti-glycation assay models. Results revealed that nimesulide (3), a non-steroidal anti-inflammatory drug, possesses a good anti-glycation activity in in-vitro BSA-MG and BSA-glucose glycation models with IC50 values of 330.56 ± 2.90, and 145.46 ± 16.35 μM, respectively. Phloroglucinol dihydrate (11), a drug used for the treatment of gastrointestinal diseases, showed a weak activity in BSA-MG glycation model (IC50 = 654.89 ± 2.50 μM), while it showed a good activity in BSA-glucose assay (IC50 = 148.23 ± 0.15 μM). Trimethylphloroglucinol (9), a drug used for the treatment of pain related to functional disorders of the digestive and biliary tracts, also showed a good antiglycation activity in BSA-MG model (IC50 = 321.15 ± 1.26 μM), while it was found to be inactive in in-vitro BSA-glucose assay (IC50 = 12.95% inhibition). These activities of drugs were compared with the anti-glycation activity of the standard, rutin (IC50 = 294.5 ± 1.50 μM in BSA-MG glycation model, and IC50 = 86.94 ± 0.24 μM in BSA- glucose model). Rest of the drugs exhibited a relatively weak antiglycation activity.
CONCLUSIONS:
This study identifies nimesulide (3), and Phloroglucinol dihydrate (11) as new inhibitors of in-vitro protein glycation for further investigations as potential anti-diabetic agents. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)