Michael reaction acceptors (MRAs) are a class of active molecules that are directly or indirectly involved in various cellular processes, including the regulation of many signaling pathways. In this study, the inducible nitric oxide synthase (iNOS) assay was used to demonstrate that the dichloromethane extract of Physalis alkekengi var. franchetii (DCEP) possesses anti-inflammatory activity that might be attributed to the modification of key cysteine residues in IKKβ by the MRAs in DCEP.
METHODS AND RESULTS:
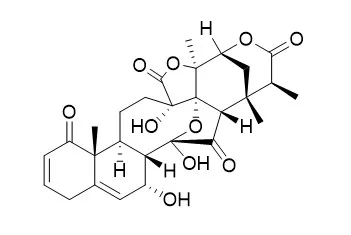
To isolate these MRAs, glutathione (GSH) was employed, and a simple ultra-performance liquid chromatography/tandem mass spectrometry (UPLC-MS/MS) screening method was developed to investigate the GSH conjugates with potential MRAs. Five physalins, including one new compound isophysalin A (2), together with four known steroidal compounds, physalin A (1), Physalin O (3), physalin L (4) and physalin G (5), were isolated to evaluate the GSH conjugating abilities, and it was indicated that compounds 1, 2 and 3, which had a common α,β-unsaturated ketone moiety, exhibited conjugating abilities with GSH and also showed significant nitric oxide (NO) production inhibiting activities.
CONCLUSIONS:
The anti-inflammatory activities of compounds 1, 2 and 3 might be attributed to their targeting multiple cysteine residues on IKKβ; therefore, the alkylation of IKKβ by compound 1 was further studied by micrOTOF-MS. The result showed that six cysteine residues (C(59), C(179), C(299), C(370), C(412), and C(618)) were alkylated, which indicated that IKKβ is a potential target for the anti-inflammatory activity of physalin A. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)