| Structure Identification: |
| Mendeleev Communications,2013,23(1);37–38. | | Aryloxyacetamides Derived from Resveratroloside and Pinostilbenoside[Reference: WebLink] |
METHODS AND RESULTS:
Etherification of phenolic hydroxyl groups of resveratroloside and Pinostilbenoside (natural 3,4’,5-trihydroxystilbene derivatives) with methyl bromoacetate afforded compounds ArOCH2CO2Me, which on treatment with amines produce the corresponding ‘stilbenyl- oxyacetamides’ in good yields. | | Chemistry of Natural Compounds,2012,48(1): 1-7. | | Reaction of several resveratrol glycoside derivatives with hypochlorites in various media[Reference: WebLink] |
METHODS AND RESULTS:
The reactions of pterostilbenoside (trans-3,5-dimethoxystilben-4′-O-β-D-glucoside) and Ar–O–Tr derivatives of resveratroloside (3,5-dihydroxystilben-4′-O-β-D-glucoside) and Pinostilbenoside (3-methoxy-5-hydroxystilben-4′-O-β-D-glucoside) with NaOCl and t-BuOCl in the presence of the stable nitroxyl radical TEMPO were studied in various media. It was found that the principal product of pterostilbenoside transformation was its 2,6-dichloroderivative, a part of which was oxidized to form 2,6-dichloropterostilbene glucuronide.
CONCLUSIONS:
Trityl ethers of resveratroloside and Pinostilbenoside reacted with the hypochlorites to form mixtures of products. | | Chemistry of Natural Compounds,1975,11( 6):715-719. | | Hydroxystilbenes of the inner bark of Pinus sibirica[Reference: WebLink] |
METHODS AND RESULTS:
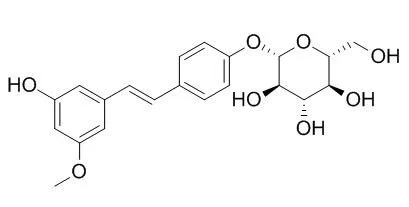
In addition to pinostilbene and resveratrol, two new stilbene glycosides have been isolated from the phloem ofPinus sibirica R. Mayr, and their structures have been established as 3,4′-dihydroxy-5-methoxy-stilbene 4′-β-D-glycopyranoside (Pinostilbenoside) and 3,4′,5-trihydroxystilbene 4′-β-D-glycopyranoside (resveratroloside). |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)