| In vitro: |
| PLoS One. 2015 May 14;10(5):e0126481. | | Antimicrobial Air Filters Using Natural Euscaphis japonica Nanoparticles.[Pubmed: 25974109] | Controlling bioaerosols has become more important with increasing participation in indoor activities. Treatments using natural-product nanomaterials are a promising technique because of their relatively low toxicity compared to inorganic nanomaterials such as silver nanoparticles or carbon nanotubes.
METHODS AND RESULTS:
In this study, antimicrobial filters were fabricated from natural Euscaphis japonica nanoparticles, which were produced by nebulizing E. japonica extract. The coated filters were assessed in terms of pressure drop, antimicrobial activity, filtration efficiency, major chemical components, and cytotoxicity. Pressure drop and antimicrobial activity increased as a function of nanoparticle deposition time (590, 855, and 1150 μg/cm2(filter) at 3-, 6-, and 9-min depositions, respectively). In filter tests, the antimicrobial efficacy was greater against Staphylococcus epidermidis than Micrococcus luteus; ~61, ~73, and ~82% of M. luteus cells were inactivated on filters that had been coated for 3, 6, and 9 min, respectively, while the corresponding values were ~78, ~88, and ~94% with S. epidermidis. Although statistically significant differences in filtration performance were not observed between samples as a function of deposition time, the average filtration efficacy was slightly higher for S. epidermidis aerosols (~97%) than for M. luteus aerosols (~95%). High-performance liquid chromatography (HPLC) and electrospray ionization-tandem mass spectrometry (ESI/MS) analyses confirmed that the major chemical compounds in the E. japonica extract were 1(ß)-O-galloyl pedunculagin, Quercetin-3-O-glucuronide, and kaempferol-3-O-glucoside. In vitro cytotoxicity and disk diffusion tests showed that E. japonica nanoparticles were less toxic and exhibited stronger antimicrobial activity toward some bacterial strains than a reference soluble nickel compound, which is classified as a human carcinogen.
CONCLUSIONS:
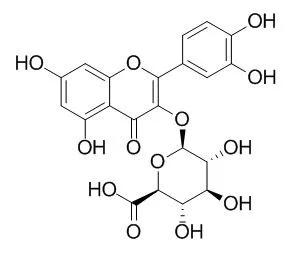
This study provides valuable information for the development of a bioaerosol control system that is environmental friendly and suitable for use in indoor environments. | | Arch Biochem Biophys . 2014 Sep 1;557:18-27. | | Quercetin-3-O-glucuronide inhibits noradrenaline-promoted invasion of MDA-MB-231 human breast cancer cells by blocking β₂-adrenergic signaling[Pubmed: 24929186] | | Abstract
Endogenous catecholamines such as adrenaline (A) and noradrenaline (NA) are released from the adrenal gland and sympathetic nervous system during exposure to stress. The adrenergic system plays a central role in stress signaling, and excessive stress was found to be associated with increased production of reactive oxygen species (ROS). Overproduction of ROS induces oxidative damage in tissues and causes the development of diseases such as cancer. In this study, we investigated the effects of Quercetin-3-O-glucuronide (Q3G), a circulating metabolite of quercetin, which is a type of natural flavonoid, on the catecholamine-induced β2-adrenergic receptor (β2-AR)-mediated response in MDA-MB-231 human breast cancer cells expressing β2-AR. Treatment with A or NA at concentrations above 1μM generated significant levels of ROS, and NA treatment induced the gene expression of heme oxygenase-1 (HMOX1), and matrix metalloproteinase-2 (MMP-2) and -9 (MMP9). Inhibitors of p38 MAP kinase (SB203580), cAMP-dependent protein kinase (PKA) (H-89), activator protein-1 (AP-1) transcription factor (SR11302), and NF-κB and AP-1 (Tanshinone IIA) decreased MMP2 and MMP9 gene expression. NA also enhanced cAMP induction, RAS activation and phosphorylation of ERK1/2. These results suggested that the cAMP-PKA, MAPK, and ROS-NF-κB pathways are involved in β2-AR signaling. Treatment with 0.1μM Q3G suppressed ROS generation, cAMP and RAS activation, phosphorylation of ERK1/2 and the expression of HMOX1, MMP2, and MMP9 genes. Furthermore, Q3G (0.1μM) suppressed invasion of MDA-MB-231 breast cancer cells and MMP-9 induction, and inhibited the binding of [(3)H]-NA to β2-AR. These results suggest that Q3G may function to suppress invasion of breast cancer cells by controlling β2-adrenergic signaling, and may be a dietary chemopreventive factor for stress-related breast cancer. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)