| In vitro: |
| Cancer Res. 2003 Aug 15;63(16):4984-9. | | Procaine is a DNA-demethylating agent with growth-inhibitory effects in human cancer cells.[Pubmed: 12941824] | Methylation-associated silencing of tumor suppressor genes is recognized as being a molecular hallmark of human cancer. Unlike genetic alterations, changes in DNA methylation are potentially reversible. This possibility has attracted considerable attention from a therapeutics standpoint. Nucleoside-analogue inhibitors of DNA methyltransferases, such as 5-aza-2'-deoxycytidine, are able to demethylate DNA and restore silenced gene expression. Unfortunately, the clinical utility of these compounds has not yet been fully realized, mainly because of their side effects. A few non-nucleoside inhibitors of DNA methyltransferases have been reported, including the anti-arrhythmia drug procainamide. Following this need to find new demethylating agents, we have tested the potential use of Procaine, an anesthetic drug related to procainamide.
METHODS AND RESULTS:
Using the MCF-7 breast cancer cell line, we have found that Procaine is a DNA-demethylating agent that produces a 40% reduction in 5-methylcytosine DNA content as determined by high-performance capillary electrophoresis or total DNA enzyme digestion. Procaine can also demethylate densely hypermethylated CpG islands, such as those located in the promoter region of the RAR beta 2 gene, restoring gene expression of epigenetically silenced genes. This property may be explained by our finding that Procaine binds to CpG-enriched DNA. Finally, Procaine also has growth-inhibitory effects in these cancer cells, causing mitotic arrest.
CONCLUSIONS:
Thus, Procaine is a promising candidate agent for future cancer therapies based on epigenetics. | | Anesth Analg. 2003 Jul;97(1):85-90, | | Procaine and mepivacaine have less toxicity in vitro than other clinically used local anesthetics.[Pubmed: 12818948] | | The neurotoxicity of local anesthetics can be demonstrated in vitro by the collapse of growth cones and neurites in cultured neurons. We compared the neurotoxicity of Procaine, mepivacaine, ropivacaine, bupivacaine, lidocaine, tetracaine, and dibucaine by using cultured neurons from the freshwater snail Lymnaea stagnalis. A solution of local anesthetics was added to the culture dish to make final concentrations ranging from 1 x 10(-6) to 2 x 10(-2) M. Morphological changes in the growth cones and neurites were observed and graded 1 (moderate) or 2 (severe). The median concentrations yielding a score of 1 were 5 x 10(-4) M for Procaine, 5 x 10(-4) M for mepivacaine, 2 x 10(-4) M for ropivacaine, 2 x 10(-4) M for bupivacaine, 1 x 10(-4) M for lidocaine, 5 x 10(-5) M for tetracaine, and 2 x 10(-5) M for dibucaine. Statistically significant differences (P < 0.05) were observed between mepivacaine and ropivacaine, bupivacaine and lidocaine, lidocaine and tetracaine, and tetracaine and dibucaine. The order of neurotoxicity was Procaine = mepivacaine < ropivacaine = bupivacaine < lidocaine < tetracaine < dibucaine. Although lidocaine is more toxic than bupivacaine and ropivacaine, mepivacaine, which has a similar pharmacological effect to lidocaine, has the least-adverse effects on cone growth among clinically used local anesthetics. |
|


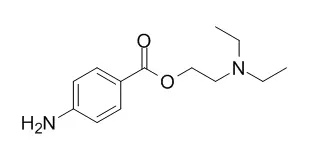



 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)