| Kinase Assay: |
| J Biol Chem, 2007 May 4;282(18):13395-401. | | The rapamycin-binding domain of the protein kinase mammalian target of rapamycin is a destabilizing domain.[Pubmed: 17350953] | Rapamycin is an immunosuppressive drug that binds simultaneously to the 12-kDa FK506- and rapamycin-binding protein (FKBP12, or FKBP) and the FKBP-rapamycin binding (FRB) domain of the mammalian target of rapamycin (mTOR) kinase. The resulting ternary complex has been used to conditionally perturb protein function, and one such method involves perturbation of a protein of interest through its mislocalization.
METHODS AND RESULTS:
We synthesized two rapamycin derivatives that possess large substituents at the C-16 position within the FRB-binding interface, and these derivatives were screened against a library of FRB mutants using a three-hybrid assay in Saccharomyces cerevisiae. Several FRB mutants responded to one of the rapamycin derivatives, and twenty of these mutants were further characterized in mammalian cells. The mutants most responsive to the ligand were fused to yellow fluorescent protein, and fluorescence levels in the presence and absence of the ligand were measured to determine stability of the fusion proteins. Wild-type and mutant FRB domains were expressed at low levels in the absence of the rapamycin derivative, and expression levels rose up to 10-fold upon treatment with ligand. The synthetic rapamycin derivatives were further analyzed using quantitative mass spectrometry, and one of the compounds was found to contain contaminating rapamycin. Furthermore, uncontaminated analogs retained the ability to inhibit mTOR, although with diminished potency relative to rapamycin.
CONCLUSIONS:
The ligand-dependent stability displayed by wild-type FRB and FRB mutants as well as the inhibitory potential and purity of the rapamycin derivatives should be considered as potentially confounding experimental variables when using these systems. |
|
| Cell Research: |
| Cancer Res,2005 Apr 15;65(8):3336-46. | | Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors.[Pubmed: 15833867] | Cell lines:U87-MG, T98G, and U373-MG
Concentrations: Dissolved in DMSO, final concentrations ~25 μM
Incubation Time: 72 hours
Method:
Cells are exposed to various concentrations of Rapamycin for 72 hours. For the assessment of cell viability, cells are collected by trypsinization, stained with trypan blue, and the viable cells in each well are counted. For the determination of cell cycle, cells are trypsinized, fixed with 70% ethanol, and stained with propidium iodide using a flow cytometry reagent set. Samples are analyzed for DNA content using a FACScan flow cytometer and CellQuest software. For apoptosis detection, cells are stained with the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) technique using an ApopTag apoptosis detection kit. To detect the development of acidic vesicular organelles (AVO), cells are stained with acridine orange (1 μg/mL) for 15 minutes, and examined under a fluorescence microscope. To quantify the development of AVOs, cells are stained with acridine orange (1 μg/mL) for 15 minutes, removed from the plate with trypsin-EDTA, and analyzed using the FACScan flow cytometer and CellQuest software. To analyze the autophagic process, cells are incubated for 10 minutes with 0.05 mM monodansylcadaverine at 37 °C and are then observed under a fluorescence microscope.
|
|


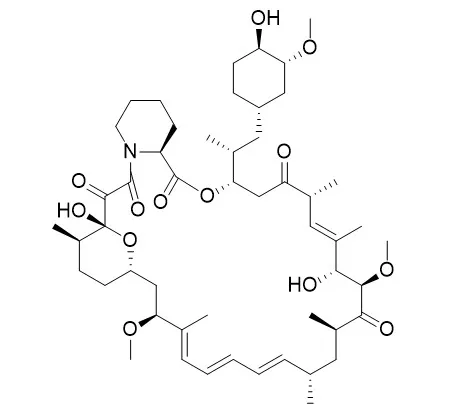



 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)