| Structure Identification: |
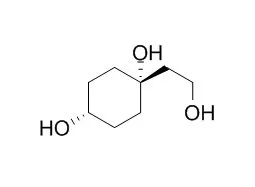
| Tetrahedron.2006 May;62(2): 4823–4828. | | Chemo enzymatic synthesis of Rengyol and Isorengyol.[Reference: WebLink] | Cyanohydrins 2 of O-protected 4-hydroxycyclohexanones 1 are excellent starting compounds for the synthesis of IsoRengyol (I) and Rengyol (II).
METHODS AND RESULTS:
The cyano group of the O-benzyl derivative 2d is first converted into the corresponding aldehyde 4, which via Wittig olefination led to the vinyl compound 6. Hydroboration of the trans-derivative (trans-6) leads, after debenzylation, to IsoRengyol, whereas hydroboration and debenzylation of the cis-isomer (cis-6) gives Rengyol. With hydroxynitrile lyases (HNLs) as catalysts the stereoselective preparation of cis- as well as trans-cyanohydrin 2d is possible, which enables the selective preparation of IsoRengyol or Rengyol, respectively. The trans-configuration of IsoRengyol and the cis-configuration of Rengyol were secured by X-ray crystal structure analysis. | | Phytochemistry Letters, 2014, 7(2):111-3. | | New cyclohexylethanoids from the leaves of Clerodendrum trichotomum[Reference: WebLink] |
METHODS AND RESULTS:
A series of cyclohexylethanoids, including two new compounds, 1-hydroxy-1-(8-palmitoyloxyethyl) cyclohexanone (1) and 5-O-butyl cleroindin D (2), together with five known ones, Rengyolone (3), cleroindin C (4), cleroindin B (5), Rengyol (6) and isoRengyol (7), were isolated from the leaves of Clerodendrum trichotomum. Their structures were elucidated on the basis of spectroscopic analyses and by comparison of their NMR data with those in the literatures. Compounds 1 and 2 were evaluated for their cytotoxicity against A549 human tumor cell line. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)