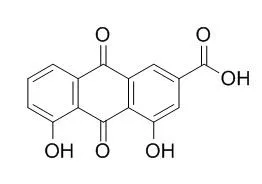
| Description: |
Rhein has many pharmacological effects, including epatoprotective, nephroprotective, anti-inflammatory, antioxidant, anticancer, and antimicrobial activities, it has been proved effective in treatment of experimental diabetic nephropathy , one of the mechanism is the Inhibition of the hexosamine pathway. Rhein has protective effect on liver injury, the mechanisms possibly contribute to its action of antioxidant and anti-inflammatory activity, also associated with its effect of inhibiting TGF-β1 and suppressing the activation of hepatic stellate cells. |
| In vitro: |
| Phytother Res. 2015 Mar;29(3):407-14. | | Anti-fibrotic and Anti-tumorigenic Effects of Rhein, a Natural Anthraquinone Derivative, in Mammalian Stellate and Carcinoma Cells.[Pubmed: 25510440] | Anthraquinone compounds have been recognized to possess antiinflammatory, anti-fibrotic and anti-tumour properties and thus applied in human and veterinary therapeutics as active substances of medicinal products. Amongst the anthraquinones isolated from Rheum palmatum, also known as da-huang, Rhein was detected as one of the highest metabolite contents in the bloodstream of mammals. The biological activities of Rhein therefore deserve detailed investigation.
METHODS AND RESULTS:
In this study, we aimed to delineate the mechanism of inhibitory actions of Rhein on fibrotic and tumorigenic processes by means of various biochemical assays, such as immunofluorescent staining, real-time polymerase chain reaction (PCR) and western blotting analyses in rat pancreatic stellate cells (LTC-14), human pancreatic ductal adenocarcinoma cells (PANC-1) and human colon carcinoma cells (SW480 and SW620). Our results demonstrated that the application of Rhein notably suppressed the mRNA and protein levels of various fibrotic and tumorigenic mediators including alpha-smooth muscle actin, type I collagen, fibronectin, N-cadherin and matrix metalloproteinases in the testing mammalian cells. The mechanism of the suppressive actions of Rhein was associated with the modulation of the sonic hedgehog and serine-threonine kinase signalling pathways.
CONCLUSIONS:
In conclusion, we suggest that Rhein may serve as a therapeutic or an adjuvant agent in anti-fibrotic and anti-tumorigenic approaches. | | Inflammation, 2003, 27(4):233-46. | | Rhein inhibits interleukin-1 beta-induced activation of MEK/ERK pathway and DNA binding of NF-kappa B and AP-1 in chondrocytes cultured in hypoxia: a potential mechanism for its disease-modifying effect in osteoarthritis.[Pubmed: 14527176] |
METHODS AND RESULTS:
In the present report, we show that bovine articular chondrocytes cultured in low oxygen tension, i.e. in conditions mimicking their hypoxic in vivo environment, respond to IL-1beta (10 ng/mL) by an increased DNA binding activity of NF-kappaB and AP-1 transcription factors. Incubation of the cells with 10(-5) M Rhein for 24 h was found to reduce this activity, particularly in the case of AP-1. Mitogen activated kinases (ERK-1 and ERK-2) were activated by exposure of the chondrocytes to 1-h treatment with IL-1beta. This effect was greater in hypoxia (3% O2) than in normoxia (21% O2). Rhein was capable of reducing the IL-1beta-stimulated ERK1/ERK2 pathway whatever the tension of oxygen present in the environment. The level of c-jun protein, an element of AP-1 complex, was increased by exposure of the chondrocytes to IL-1beta after 2, 6, and 24 h. Addition of Rhein at 10(-5) M for 24 h did not reduce the c-jun protein amount. The mRNA steady-state levels of collagen type II (COL2A1) and aggrecan core protein were found to be significantly increased by a 24-h treatment with 10(-5) M Rhein. This stimulating effect was also observed in the presence of IL-1beta, suggesting that the drug could prevent or reduce the IL-1beta-induced inhibition of extracellular matrix synthesis. IL-1-induced collagenase (MMPI) expression was significantly decreased by Rhein in the same conditions. In conclusion, Rhein can effectively inhibit the IL-1-activated MAPK pathway and the binding of NF-kappaB and AP-1 transcription factors, two key factors involved in the expression of several proinflammatory genes by chondrocytes. In addition, the drug can reduce the procatabolic effect of the cytokine, by reducing the MMPI synthesis, and enhance the synthesis of matrix components, such as type II collagen and aggrecan.
CONCLUSIONS:
These results may explain the antiosteoarthritic properties of Rhein and its disease-modifying effects on OA cartilage, in spite of absence of inhibition at prostaglandin level. |
|
| In vivo: |
| Acta Pharmacol Sin. 2002 Aug;23(8):739-44. | | Rhein inhibits liver fibrosis induced by carbon tetrachloride in rats.[Pubmed: 12147197] | To investigate the effect of Rhein on liver fibrosis induced by the exposure of carbon tetrachloride (CCl4)/ethanol in rats.
METHODS AND RESULTS:
Male Wistar rats were divided into four study groups (n=10 each group): healthy controls, CCl4/ethanol-injured rats left untreated, and CCl4/ethanol-injured rats treated with Rhein of low-dose (25 mg/kg) and high-dose (100 mg/kg). Rhein was given once a day since rat received CCl4/ethanol injury. After administration of Rhein for 6 weeks rats were killed. The following parameters were determined: the activity of alanine aminotransferase (ALT), hyalauronic acid (HA) and procollagen type III (PC-III) concentrations in serum, liver malondialdehyde (MDA) level, the degree of liver fibrosis, and the expression of alpha-smooth muscle actin (alpha-SMA) and transforming growth factor-beta1 (TGF-beta1) in liver tissue.
The treatment of Rhein markedly reduced the ALT activity, HA and PC-III concentrations, and liver MDA level in CCl4/ethanol-injured rats (P<0.01). It also improved significantly histological changes of fibrosis and decreased the expression of alpha-SMA and TGF-beta1 in liver of these rats (P<0.05 or P<0.01).
CONCLUSIONS:
Rhein has protective effect on liver injury and can inhibit liver fibrosis induced by CCl4/ethanol in rats. The mechanisms possibly contribute to its action of antioxidant and anti-inflammatory activity, also associated with its effect of inhibiting TGF-beta1 and suppressing the activation of hepatic stellate cells. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)