| In vitro: |
| J Nat Prod. 2008 Jun;71(6):1049-51. | | Tenacigenin B derivatives reverse P-glycoprotein-mediated multidrug resistance inHepG2/Dox cells.[Pubmed: 18512984] |
METHODS AND RESULTS:
Tenacissimoside A (1) and 11alpha-O-benzoyl-12beta- O-acetylTenacigenin B (2), two derivatives of Tenacigenin B (3) from the plant Marsdenia tenacissima, reversed multidrug resistance in P-glycoprotein (Pgp)-overexpressing multidrug-resistant cancer cells. The sensitivity of HepG2/Dox cells to the antitumor drugs doxorubicin, vinblastine, puromycin, and paclitexel was increased by 18-, 10-, 11-, and 6-fold by 20 microg/mL (or 25 microM) of 1 and 16-, 53-, 16-, and 326-fold by 20 microg/mL (or 39 microM) of 2, respectively.
CONCLUSIONS:
A preliminary mechanistic study has suggested that 1 might modulate Pgp-mediated multidrug resistance through directly interacting with the Pgp substrate site. | | J Nat Med. 2016 Jul;70(3):602-9. | | The cytotoxic and tyrosine kinase inhibitory properties of C21 steroids and iridoids from the tubers of Alocasia cucullata.[Pubmed: 27120176 ] |
METHODS AND RESULTS:
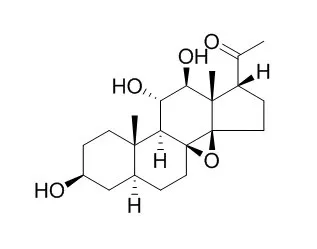
Ten steroids and iridoids were isolated from the tubers of Alocasia cucullata (Lour.) G. Don. Among them, alocasgenin A (1) and alocasgenoside B-C (2-3) were new compounds and the aglycone of compound 1, obtained from the acid hydrolysis of 1, was named alocasgenol (1a). Also, for the first time, Tenacigenin B (4), 17β-tenacigenin-B (5), 3-O-6-deoxy-3-O-methyl-β-D-allopyranosyl-(1→4)-β-D-oleandropyranosyl-tenacigenin C (6), marsdenoside A-B (7-8) and tenacigenoside A-B (9-10) were isolated from the genus Alocasia. The chemical structures were elucidated by the extensive analysis of spectral data and compared with the literature.
CONCLUSIONS:
By evaluation of the cytotoxic and tyrosine kinase inhibition, compounds 1-10, 1a and compound 2 showed significant growth inhibition against two tumour cell lines, MGC-803 and HT-29, while compounds 1, 1a, 3, 6 and 8 presented moderate inhibition. Furthermore, compound 2 had the inhibitory property against the enzyme activity biochemically. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)