| In vitro: |
| J Antibiot (Tokyo). 2014 Jan;67(1):127-32. | | Medium optimization of Streptomyces sp. 17944 for tirandamycin B production and isolation and structural elucidation of tirandamycins H, I and J.[Pubmed: 23715040] |
METHODS AND RESULTS:
We have recently isolated tirandamycin (TAM) B from Streptomyces sp. 17944 as a Brugia malayi AsnRS (BmAsnRS) inhibitor that efficiently kills the adult B. malayi parasites and does not exhibit general cytotoxicity to human hepatic cells. We now report (i) the comparison of metabolite profiles of S. sp. 17944 in six different media, (ii) identification of a medium enabling the production of Tirandamycin B as essentially the sole metabolite, and with improved titer, and (iii) isolation and structural elucidation of three new TAM congeners.
CONCLUSIONS:
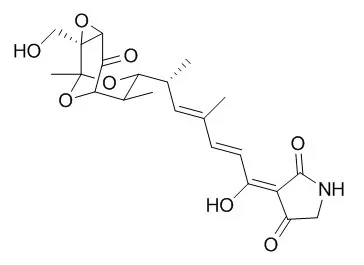
These findings shed new insights into the structure-activity relationship of Tirandamycin B as a BmAsnRS inhibitor, highlighting the δ-hydroxymethyl-α,β-epoxyketone moiety as the critical pharmacophore, and should greatly facilitate the production and isolation of sufficient quantities of Tirandamycin B for further mechanistic and preclinical studies to advance the candidacy of Tirandamycin B as an antifilarial drug lead.
The current study also serves as an excellent reminder that traditional medium and fermentation optimization should continue to be very effective in improving metabolite flux and titer. | | Org Lett. 2015 Feb 6;17(3):628-31. | | Discovery of a new family of Dieckmann cyclases essential to tetramic acid and pyridone-based natural products biosynthesis.[Pubmed: 25621700] |
METHODS AND RESULTS:
Bioinformatic analyses indicate that TrdC, SlgL, LipX2, KirHI, and FacHI belong to a group of highly homologous proteins involved in biosynthesis of actinomycete-derived Tirandamycin B, streptolydigin, α-lipomycin, kirromycin, and factumycin, respectively. However, assignment of their biosynthetic roles has remained elusive.
CONCLUSIONS:
Gene inactivation and complementation, in vitro biochemical assays with synthetic analogues, point mutations, and phylogenetic tree analyses reveal that these proteins represent a new family of Dieckmann cyclases that drive tetramic acid and pyridone scaffold biosynthesis. | | Chinese Journal of Marine Drugs, 2010(6):12-20. | | Fermentation optimization,isolation and identification of tirandamycins A and B from marine-derived Streptomyces sp.SCSIO 1666.[Reference: WebLink] | To screen antitumor and antibacterial agents from marine actinomycetes isolated from marine sediment,and strain Streptomyces sp.SCSI01666 was selected for further fermentation optimization to produce bioactive secondary metabolites.
METHODS AND RESULTS:
Bioassay was conducted using in vitro antitumor activities against six tumor cell lines A549,DU145,H1299,HCT15,HEP3B,SF268,and using antibacterial models.The fermentation conditions of the strain were optimized and the bioactive secondary metabolites were isolated by means of solvent extraction,normal and reversed-phase silica gel.The structures of compounds were identified by physicochemical properties and spectral analyses.
CONCLUSIONS:
Two bioactive compounds were isolated guided by HPLC-UV and bioassay against Bacillus subtilis and their structures were identified as tirandamycin A(1) and Tirandamycin B (2) from strain SCSI01666.The production of the two bioactive compounds was found to be marine salt dependent and there was only trace amount of compounds produced if no marine salt was added.The fermentation titer of the two compounds was improved more than 250-fold when 3%marine salt was added to the medium.Also,the fermentation titer of tirandamycin A was found to be greatly improved by addition of XAD-16 resin. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)