| In vitro: |
| Agricultural & Biological Chemistry, 2006 , 54 (9) :2367-71. | | Turmeronol A and turmeronol B, new inhibitors of soybean lipoxygenase[Reference: WebLink] |
METHODS AND RESULTS:
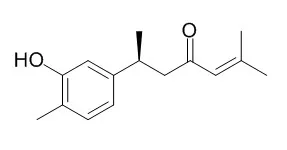
In the course of our screening program for inhibitors of soybean lipoxygenase, we isolated two new phenolic sesquiterpene ketones named Turmeronol A and turmeronol B from the spice turmeric (dried rhizome of Curucuma longa L.). Their structures were determined to be 2-methyl-6-(3-hydroxy-4-methylphenyl)-2-hepten-4-one and 2-methyl-6-(2-hydroxy-4-methylphenyl)-2-hepten-4-one, respectively.
CONCLUSIONS:
Turmeronol A and turmeronol B inhibited soybean lipoxygenase at the IC50 values of 16 µm and 9 µm, respectively. These compounds prevented the autoxidation of linoleic acid at a concentration of ca. 200 ppm. | | Dhaka University Journal of Pharmaceutical Sciences, 2010, 8(1):39-45. | | Phytochemical and Biological Investigations of Curcuma longa.[Reference: WebLink] |
METHODS AND RESULTS:
Seven compounds, Turmeronol A (1), turmeronol B (2), 3,4-dimethoxycinnamic acid (3), 4-hydroxy- 3-methoxycinnamic acid (4), 4-hydroxybenzaldehyde (5), 2,3,5,6-tetrahydroxyarturmerone (6) and 4-hydroxy- bisabola-2,10-diene-9-one (7) have been isolated from the carbon tetrachloride soluble fraction of a methanol extract of the rhizomes of Curcuma longa. Careful interpretation of the NMR data and comparison with Turmeronol A (1) allowed tentative identification of compound 6 as 2,3,5,6-tetrahydroxy-arturmerone, which appears to be a new molecule.
CONCLUSIONS:
The n-hexane, carbon tetrachloride and chloroform extractives of the rhizomes when subjected to antimicrobial screening and brine shrimp lethality bioassay demonstrated mild to moderate antimicrobial activity and strong cytotoxicity with LC50 value 1.56 μg/ml. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)