| Kinase Assay: |
| Biomed Chromatogr. 2015 May 29. | | Hepatic, gastric and intestinal first-pass effects of vitexin-2″-O-rhamnoside in rats by ultra-high performance liquid chromatography.[Pubmed: 26031900 ] | Previous research in our laboratory found that the absolute bioavailability of Vitexin-2''-O-rhamnoside (VR) was quite low at 4.89%.
METHODS AND RESULTS:
A rapid and sensitive UHPLC method using hesperidin as an internal standard was therefore developed and validated to investigate the reasons for this by determining VR in rat plasma after administering intravenously, intraportally (5 mg/kg), intraduodenally and intragastrically (40 mg/kg) to the rat model of the hepatic, gastric and intestinal first-pass effects. As only a high intestinal first-pass effect of VR was found, that is, there existed a low bioavailability of VR (2.40%), inhibitors of P-glycoprotein (P-gp) and cytochrome P450 3A (CYP3A), including verapamil, cyclosporin A and midazolam, and absorption enhancers, including bile salts and borneol, combined with VR, were instilled into duodenum to evaluate the effects on bioavailability of VR.
CONCLUSIONS:
The results demonstrated that area under the concentration-time curve (AUC) values of VR slightly increased after administration of verapamil, cyclosporin A and midazolam, indicating that CYP3A and P-gp do not play an important role in the first-pass effect in the intestine. AUC values of VR significantly increased after administering bile salts or borneol, indicating that the low bioavailability of VR was mainly related to its poor absorption in the intestine. |
|
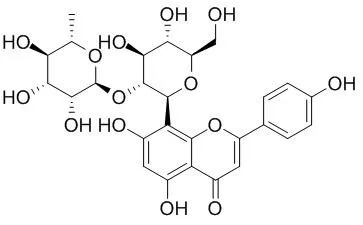
| Structure Identification: |
| J Chromatogr B Analyt Technol Biomed Life Sci. 2010 Jul 1;878(21):1837-44. | | Simultaneous determination of vitexin-4''-O-glucoside, vitexin-2''-O-rhamnoside, rutin and vitexin from hawthorn leaves flavonoids in rat plasma by UPLC-ESI-MS/MS.[Pubmed: 20570577] |
METHODS AND RESULTS:
A sensitive and accurate ultra-performance liquid chromatography electrospray ionization tandem mass spectrometry (UPLC-ESI-MS/MS) method was developed and validated for the simultaneous determination of vitexin-4''-O-glucoside (VGL), Vitexin-2''-O-rhamnoside (VRH), rutin (RUT) and vitexin (VIT) in rat plasma after intravenous administration of hawthorn leaves flavonoids (HLF). Following protein precipitation by methanol, the analytes were separated on an ACQUITY UPLC BEH C(18) column packed with 1.7 microm particles by gradient elution using a mobile phase composed of acetonitrile and water (containing 0.1% formic acid) at a flow rate of 0.20 mL/min. The analytes and diphenhydramine (internal standard, IS) were detected in the multiple reaction monitoring (MRM) mode by means of an electrospray ionization (ESI) interface (m/z 292.96 for vitexin-4''-O-glucoside, m/z 293.10 for Vitexin-2''-O-rhamnoside, m/z 299.92 for rutin, m/z 310.94 for vitexin and m/z 166.96 for IS).
The calibration curve was linear over the range 10-40,000 ng/mL for vitexin-4''-O-glucoside, 10-50,000 ng/mL for Vitexin-2''-O-rhamnoside, 8-1000 ng/mL for rutin and 16-2000 ng/mL for vitexin. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)