| In vitro: |
| Fitoterapia. 2012 Sep;83(6):1076-80. | | Chemical constituents of Anacolosa pervilleana and their antiviral activities.[Pubmed: 22613073] | In an effort to identify novel inhibitors of Chikungunya (CHIKV) and Dengue (DENV) virus replication, a systematic study with 820 ethyl acetate extracts of Madagascan plants was performed in a virus-cell-based assay for CHIKV and a DENV NS5 RNA-dependant RNA polymerase (RdRp) assay.
METHODS AND RESULTS:
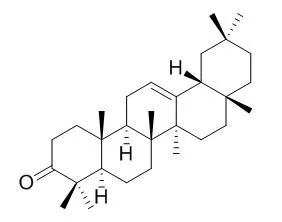
The extract obtained from the leaves of Anacolosa pervilleana was selected for its significant activity in both assays. One new (E)-tridec-2-en-4-ynedioic acid named anacolosine (1), together with three known acetylenic acids, the octadeca-9,11,13-triynoic acid (2), (13E)-octadec-13-en-9,11-diynoic acid (3), (13E)-octadec-13-en-11-ynoic acid (4), two terpenoids, lupenone (5) and beta-Amyrone (6), and one cyanogenic glycoside, (S)-sambunigrin (7) were isolated. Their structures were elucidated by comprehensive analyses of NMR spectroscopy and mass spectrometry data. The inhibitory potency of these compounds was evaluated on CHIKV, DENV RdRp and West-Nile polymerase virus (WNV RdRp).
CONCLUSIONS:
Both terpenoids showed a moderate activity against CHIKV (EC(50) 77 and 86 μM, respectively) and the acetylenic acids produced IC(50) values around 3 μM in the DENV RdRp assay. | | Phytochem. Lett., 2011, 4(1):34-7. | | Chemical constituents of Drypetes gossweileri and their enzyme inhibitory and anti-fungal activities.[Reference: WebLink] | Phytochemical studies on the methanolic extract of Drypetes gossweileri afforded N-β-d-glucopyranosyl-p-hydroxyphenylacetamide (1), p-hydroxyphenylacetic acid (2), p-hydroxyphenyl-acetonitrile (3), p-hydroxyacetophenone (4), 3,4,5-trimethoxyphenol (5), dolichandroside A (6), and beta-Amyrone(7).
METHODS AND RESULTS:
Compounds 1–7 were identified with the aid of extensive NMR and MS spectroscopic studies. Compound 1 was a new natural product and was isolated for the first time from plant containing N-glucose moiety incorporated in its structure. Compounds 1–7 exhibited moderate to the weak source anti-α-glucosidase inhibitory activity. Compound 7 exhibited moderate anti-acetylcholinesterase (AChE) activity while the rest of the isolates were weakly active in this bioassay. Compounds 1–7 also showed moderate anti-fungal activity.Graphical abstractFrom the methanolic extract of Drypetes gossweileri, one new natural product, N-β-d-glucopyranosyl-p-hydroxyphenylacetamide (1), along with six known natural products, p-hydroxyphenylacetic acid (2), p-hydroxyphenylacetonitrile (3), p-hydroxyacetophenone (4), 3,4,5-trimethoxyphenol (5), dolichandroside A (6), and beta-Amyrone (7) were isolated. All of the isolates exhibited different levels of anti-α-glucosidase, anti-acetylcholinesterase and anti-fungal activities.Research highlightsChemical investigation of Drypetes gossweileri afforded one new natural product, N-β-d-glucopyranosyl-p-hydroxyphenylacetamide (1), along with six known natural products.
CONCLUSIONS:
All of the isolates exhibited different levels of anti-α-glucosidase, anti-acetylcholinesterase and anti-fungal activities. | | PLoS Negl Trop Dis. 2015 Mar; 9(3): e0003488. | | The In Vitro Antifungal Activity of Sudanese Medicinal Plants against Madurella mycetomatis, the Eumycetoma Major Causative Agent.[Pubmed: 25768115] |
Eumycetoma is a debilitating chronic inflammatory fungal infection that exists worldwide but it is endemic in many tropical and subtropical regions. The major causative organism is the fungus Madurella mycetomatis. The current treatment of eumycetoma is suboptimal and characterized by low cure rate and high recurrence rates. Hence, an alternative therapy is needed to address this.
METHODS AND RESULTS:
Here we determined the antifungal activity of seven Sudanese medicinal plant species against Madurella mycetomatis. Of these, only three species; Boswellia papyrifera, Acacia nubica and Nigella sativa, showed some antifungal activity against M. mycetomatis and were further studied. Crude methanol, hexane and defatted methanol extracts of these species were tested for their antifungal activity. B. papyrifera had the highest antifungal activity (MIC50 of 1 ug/ml) and it was further fractionated. The crude methanol and the soluble ethyl acetate fractions of B. papyrifera showed some antifungal activity. The Gas-Liquid-Chromatography hybrid Mass-Spectrophotometer analysis of these two fractions showed the existence of beta-amyrin, beta-Amyrone, beta-Sitosterol and stigmatriene. Stigmatriene had the best antifungal activity, compared to other three phytoconstituents, with an MIC-50 of 32 μg/ml.
CONCLUSIONS:
Although the antifungal activity of the identified phytoconstituents was only limited, the antifungal activity of the complete extracts is more promising, indicating synergism. Furthermore these plant extracts are also known to have anti-inflammatory activity and can stimulate wound-healing; characteristics which might also be of great value in the development of novel therapeutic drugs for this chronic inflammatory disease. Therefore further exploration of these plant species in the treatment of mycetoma is encouraging. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)