| In vitro: |
| Appl Environ Microbiol. 2004 Jun;70(6):3222-31. | | Saturation mutagenesis of Burkholderia cepacia R34 2,4-dinitrotoluene dioxygenase at DntAc valine 350 for synthesizing nitrohydroquinone, methylhydroquinone, and methoxyhydroquinone.[Pubmed: 15184115] |
METHODS AND RESULTS:
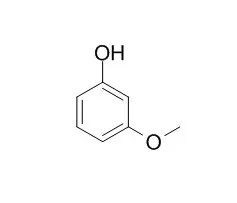
Saturation mutagenesis of the 2,4-dinitrotoluene dioxygenase (DDO) of Burkholderia cepacia R34 at position valine 350 of the DntAc alpha-subunit generated mutant V350F with significantly increased activity towards o-nitrophenol (47 times), m-nitrophenol (34 times), and o-methoxyphenol (174 times) as well as an expanded substrate range that now includes m-Methoxyphenol, o-cresol, and m-cresol (wild-type DDO had no detectable activity for these substrates). Another mutant, V350M, also displays increased activity towards o-nitrophenol (20 times) and o-methoxyphenol (162 times) as well as novel activity towards o-cresol. Products were synthesized using whole Escherichia coli TG1 cells expressing the recombinant R34 dntA loci from pBS(Kan)R34, and the initial rates of product formation were determined at 1 mM substrate by reverse-phase high-pressure liquid chromatography. V350F produced both nitrohydroquinone at a rate of 0.75 +/- 0.15 nmol/min/mg of protein and 3-nitrocatechol at a rate of 0.069 +/- 0.001 nmol/min/mg of protein from o-nitrophenol, 4-nitrocatechol from m-nitrophenol at 0.29 +/- 0.02 nmol/min/mg of protein, methoxyhydroquinone from o-methoxyphenol at 2.5 +/- 0.6 nmol/min/mg of protein, methoxyhydroquinone from m-Methoxyphenol at 0.55 +/- 0.02 nmol/min/mg of protein, both methylhydroquinone at 1.52 +/- 0.02 nmol/min/mg of protein and 2-hydroxybenzyl alcohol at 0.74 +/- 0.05 nmol/min/mg of protein from o-cresol, and methylhydroquinone at 0.43 +/- 0.1 nmol/min/mg of protein from m-cresol. V350M produced both nitrohydroquinone at a rate of 0.33 nmol/min/mg of protein and 3-nitrocatechol at 0.089 nmol/min/mg of protein from o-nitrophenol, methoxyhydroquinone from o-methoxyphenol at 2.4 nmol/min/mg of protein, methylhydroquinone at 1.97 nmol/min/mg of protein and 2-hydroxybenzyl alcohol at 0.11 nmol/min/mg of protein from o-cresol. The DDO variants V350F and V350M also exhibited 10-fold-enhanced activity towards naphthalene (8 +/- 2.6 nmol/min/mg of protein), forming (1R,2S)-cis-1,2-dihydro-1,2-dihydroxynaphthalene.
CONCLUSIONS:
Hence, mutagenesis of wild-type DDO through active-site engineering generated variants with relatively high rates toward a previously uncharacterized class of substituted phenols for the nitroarene dioxygenases; seven previously uncharacterized substrates were evaluated for wild-type DDO, and four novel monooxygenase-like products were found for the DDO variants V350F and V350M (methoxyhydroquinone, methylhydroquinone, 2-hydroxybenzyl alcohol, and 3-nitrocatechol). |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)