| Description: |
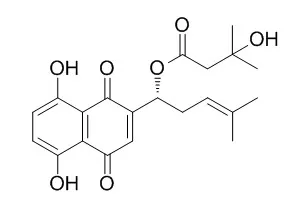
Beta-Hydroxyisovalerylshikonin is an ATP non-competitive inhibitor of protein-tyrosine kinases such as v-Src and EGFR, and has been shown to induce apoptosis in several human tumor cell lines.
Beta-Hydroxyisovalerylshikonin has anti-adipogenic, antiinflammatory and antitumor effects, it activated AMPK, which was followed by the downregulation of mature SREBP‑1c and fat-forming enzymes, leading to the inhibition of adipogenesis. Beta-Hydroxyisovalerylshikonin can significantly decrease viability of HCT116 cells (IC50 values of 30.9 ug/mL). |
| In vitro: |
| EXCLI J. 2017 Feb 16;16:73-88. | | Antibacterial and cytotoxic activities of naphthoquinone pigments from Onosma visianii Clem.[Pubmed: 28435429] | In this study, the antibacterial and cytotoxic activities of isolated compounds from the roots of Onosma visianii were investigated.
METHODS AND RESULTS:
By using different chromatographic techniques and appropriate spectroscopic methods, the seven naphthoquinones were described: deoxyshikonin ( 1 ), isobutyrylshikonin ( 2 ), α-methylbutyrylshikonin ( 3 ), acetylshikonin ( 4 ), Beta-Hydroxyisovalerylshikonin( 5 ), 5,8-O-dimethyl isobutyrylshikonin ( 6 ) and 5,8-O-dimethyl deoxyshikonin ( 7 ). Among the tested compounds, 3 and 4 exhibited the highest antibacterial activities toward all tested bacterial species (MIC50 and MIC90 for gram positive bacteria: 6.40 μg/mL-12.79 μg/mL and 6.82 μg/mL-13.60 μg/mL, respectively; for gram negative bacteria: 4.27 μg/mL-8.53 μg/mL and 4.77 μg/mL-9.54 μg/mL, respectively). Also, naphthoquinones 3 and 4 exhibited strong cytotoxic activity against MDA-MB-231 cells (IC50 values 86.0 μg/mL and 80.2 μg/mL, respectively), while compounds 1 , 3 , 4 and 5 significantly decreased viability of HCT116 cells (IC50 values of 97.8 μg/mL, 15.2 μg/mL, 24.6 μg/mL and 30.9 μg/mL, respectively).
CONCLUSIONS:
Our results indicated that all tested naphthoquinone pigments are potential candidates for clinical uses as antibacterial and cytotoxic agents. | | Int J Mol Med. 2016 Mar;37(3):816-24. | | AMPK and SREBP-1c mediate the anti-adipogenic effect of β-hydroxyisovalerylshikonin.[Pubmed: 26865314] | Beta-Hydroxyisovalerylshikonin (β-HIVS), which is a natural naphthoquinone compound, is one of the main chemicals isolated from a therapeutic plant, Lithospermum erythrorhizon.
METHODS AND RESULTS:
In the present study, we demonstrated that β-HIVS inhibited the adipogenesis of 3T3-L1 cells through AMP-activated protein kinase (AMPK)-mediated modulation of sterol regulatory element binding protein (SREBP)‑1c. The anti-adipogenic effect of β-HIVS was accompanied by the increased phosphorylation of AMPK and precursor SREBP‑1c. In β-HIVS-treated 3T3-L1 cells, AMPK was activated and phosphorylated precursor SREBP‑1c, preventing the cleavage of precursor SREBP‑1c to mature SREBP‑1c. Expression of the fat-forming enzymes, acetyl-CoA carboxylase (ACC)1, fatty acid synthase (FAS) and stearoyl-CoA desaturase (SCD)1, which are transcribed by mature SREBP‑1c, were downregulated, resulting in reduced intracellular fat accumulation. The anti-adipogenic effect of β-HIVS was significantly attenuated by AMPK knockdown. Knockdown of AMPK using siRNA decreased the phosphorylation of precursor SREBP‑1c and increased the levels of mature SREBP. The levels of the fat-forming enzymes, ACC1, FAS and SCD1, as well as intracellular fat accumulation were also significantly increased by AMPK knockdown.
CONCLUSIONS:
These results suggest that β-HIVS activated AMPK, which was followed by the downregulation of mature SREBP‑1c and fat-forming enzymes, leading to the inhibition of adipogenesis. |
|
| In vivo: |
| J Ethnopharmacol. 2012 Nov 21;144(2):335-45. | | In vitro and in vivo anticancer effects of Lithospermum erythrorhizon extract on B16F10 murine melanoma.[Pubmed: 22995444 ] | | Lithospermum erythrorhizon has long been used in traditional Asian medicine for the treatment of diseases including skin cancer. In this study, hexane extract from the roots of Lithospermum erythrorhizon (LEH) was chemically characterized and its anticancer activity was tested against the most aggressive form of skin cancer.
METHODS AND RESULTS:
The in vitro anticancer studies viz. cell growth, cell cycle and apoptosis, and the expression of tumor regulating proteins were analyzed against B16F10 melanoma cells. In addition, C57BL/6 mice models were used to evaluate the in vivo anticancer potential of LEH. Mice were intraperitoneally injected with LEH at doses of 0.1 and 10mg/kg every 3 days. The tumor inhibition ratio was determined after 21 days of treatment and the histopathological analyses of the tumor tissues were compared. Further, LEH was purified and its active compounds were structurally elucidated and identified by NMR spectra and quantified by HPLC analyses. LEH effectively inhibits the growth of melanoma cells with an IC(50) of 2.73μg/ml. Cell cycle analysis revealed that LEH increased the percentage of cells in sub-G1 phase by dose dependent manner. LEH exhibited down regulation of anti-apoptotic Bcl-2 family proteins and up regulation of apoptotic Bax protein expression. Importantly, LEH induced cleavage of poly (ADP-ribose) polymerase (PARP) and activated the caspase cascade (caspase 3) with this cleavage mediating the apoptosis of B16F10 cells. LEH treatment at a dose of 10mg/kg for 21 days in experimental mice implanted with tumors resulted in significant reduction of the tumor growth (43%) and weight (36%). Histopathology analysis of LEH treated tumor tissues showed evidence of increased necrotic cells in a concentration dependent manner. Meanwhile, five naphthoquinone compounds [Shikonin (1); Deoxyshikonin (2); Beta-Hydroxyisovalerylshikonin (3); Acetylshikonin (4) and Isobutyrylshikonin (5)] were purified from LEH and responsible for its anticancer activity.
CONCLUSIONS:
LEH induced apoptosis in B16F10 cells by activation of caspase 3 and inducing sub-G1 cell cycle arrest. LEH exhibited both in vitro and in vivo anticancer activity. Shikonin derivatives in the LEH are responsible for the anticancer activity. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)