Cholinesterase inhibition is one of the most treatment strategies against Alzheimer's disease (AD) where metal accumulation is also strongly associated with pathology of the disease.
METHODS AND RESULTS:
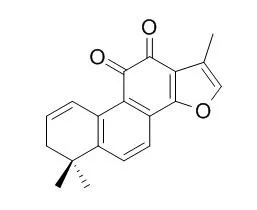
In the current study, we assessed inhibitory effect against acetyl- (AChE) and butyrylcholinesterase (BChE) and metal-chelating capacity of twelve diterpenes: arucadiol, miltirone, tanshinone IIa, 1-oxomiltirone, cryptotanshinone, 1,2-didehydromiltirone, 1,2-Didehydrotanshinone IIA, 1β-hydroxycryptotanshinone, 15,16-dihydrotanshinone, tanshinone I, isotanshinone II, 1(S)-hydroxytanshinone IIa, and rosmarinic acid, isolated from Perovskia atriplicifolia and Salvia glutinosa. The compounds were tested at 10 μg/mL using ELISA microtiter assays against AChE and BChE. QSAR and molecular docking studies have been also performed on the active compounds. All of the compounds showed higher [e.g., IC50 = 1.12 ± 0.07 μg/mL for 1,2-didehydromiltirone, IC50 = 1.15 ± 0.07 μg/mL for cryptotanshinone, IC50 = 1.20 ± 0.03 μg/mL for arucadiol, etc.)] or closer [1,2-Didehydrotanshinone IIA (IC50 = 5.98 ± 0.49 μg/mL) and 1(S)-hydroxytanshinone IIa (IC50 = 5.71 ± 0.27 μg/mL)] inhibition against BChE as compared to that of galanthamine (IC50 = 12.56 ± 0.37 μg/mL), whereas only 15,16-dihydrotanshinone moderately inhibited AChE (65.17 ± 1.39%). 1,2-Didehydrotanshinone IIA (48.94 ± 0.26%) and 1(S)-hydroxytanshinone IIa (47.18 ± 5.10%) possessed the highest metal-chelation capacity.
CONCLUSIONS:
The present study affords an evidence for the fact that selective BChE inhibitors should be further investigated as promising candidate molecules for AD therapy. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)