| Kinase Assay: |
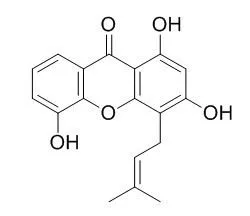
| Biochem Pharmacol. 2011 Mar 15;81(6):752-60. | | 1,3,5-trihydroxy-4-prenylxanthone represses lipopolysaccharide-induced iNOS expression via impeding posttranslational modification of IRAK-1.[Pubmed: 21232528] | 1,3,5-Trihydroxy-4-prenylxanthone (TH-4-PX) isolated from Cudrania cochinchinensis repressed lipopolysaccharide (LPS)-induced NO production in RAW264.7 macrophages.
METHODS AND RESULTS:
Here we further examined the underlying mechanisms using RT-PCR and Western blot analyses. Consistent with NO inhibition, suppression of LPS-induced iNOS expression by 1,3,5-Trihydroxy-4-prenylxanthone through abolishing IκB kinase (IKK) phosphorylation, IκB degradation and nuclear factor-κB (NF-κB) nuclear translocation was observed. After LPS stimulation, the increased nuclear level of c-Fos and c-Jun (major components of activator protein-1, AP-1) and the phosphorylated level of upstream signal molecules, such as c-Jun NH2-terminal kinase (JNK) and extracellular signal-regulated kinase, (ERK) were all significantly suppressed by 1,3,5-Trihydroxy-4-prenylxanthone, while p38 remained unaffected. A further experiment revealed that 1,3,5-Trihydroxy-4-prenylxanthone inhibited the phosphorylation of transforming growth factor-β (TGF-β)-activated kinase 1 (TAK1), an upstream signaling molecule required for IKK and mitogen-activated protein kinases (MAPKs) activation. Interestingly, the modified pattern of IRAK-1 in the presence LPS was significantly attenuated by 1,3,5-Trihydroxy-4-prenylxanthone treatment.
CONCLUSIONS:
In conclusion, 1,3,5-Trihydroxy-4-prenylxanthone inhibited LPS-induced NF-κB and AP-1 activations by interfering with the posttranslational modification (phosphorylation and/or ubiquitinylation) of IRAK-1 in the cell membrane to impede TAK1-mediated activation of IKK and MAPKs signal transduction. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)