| In vitro: |
| Phytomedicine. 2014 Sep 25;21(11):1490-6. | | Growth-inhibiting and apoptosis-inducing activities of Myricanol from the bark of Myrica rubra in human lung adenocarcinoma A549 cells.[Pubmed: 24939078 ] | This study investigated whether or not Myricanol extracted from M. rubra bark elicits anti-cancer effects on human lung adenocarcinoma A549 cells by inducing apoptosis in vivo.
METHODS AND RESULTS:
Myricanol was extracted from M. rubra bark through system solvent extraction and silica gel layer column separation. The results of tritiated thymidine assay, colony formation assay, and flow cytometry indicated that Myricanol inhibited the growth of A549 cells. The effects of Myricanol on the expression of key apoptosis-related genes in A549 cells were evaluated by quantitative PCR and Western blot analyses. Myricanol significantly inhibited the growth of A549 cells in a dose-dependent manner, with a half maximal inhibitory concentration of 4.85 μg/ml. Myricanol significantly decreased colony formation and induced A549 cell apoptosis. Myricanol upregulated the expression of Caspase-3, Caspase-9, Bax, and p21 and downregulated the expression of Bcl-2 at the mRNA and protein levels.
CONCLUSIONS:
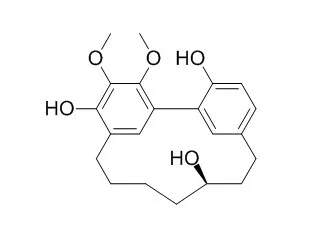
These changes were associated with apoptosis. Based on these results, we propose that Myricanol elicits growth inhibitory and cytotoxic effects on lung cancer cells. Therefore, Myricanol may be a clinical candidate for the prevention and treatment of lung cancer. | | ACS Chem Biol. 2015 Apr 17;10(4):1099-109. | | Synthesis, stereochemical analysis, and derivatization of myricanol provide new probes that promote autophagic tau clearance.[Pubmed: 25588114] | We previously discovered that one specific scalemic preparation of Myricanol (1), a constituent of Myrica cerifera (bayberry/southern wax myrtle) root bark, could lower the levels of the microtubule-associated protein tau (MAPT). The significance is that tau accumulates in a number of neurodegenerative diseases, the most common being Alzheimer's disease (AD).
METHODS AND RESULTS:
Herein, a new synthetic route to prepare Myricanol using a suitable boronic acid pinacol ester intermediate is reported. An X-ray crystal structure of the isolated Myricanol (1) was obtained and showed a co-crystal consisting of (+)-aR,11S-Myricanol (2) and (-)-aS,11R-Myricanol (3) coformers. Surprisingly, 3, obtained from chiral separation from 1, reduced tau levels in both cultured cells and ex vivo brain slices from a mouse model of tauopathy at reasonable mid-to-low micromolar potency, whereas 2 did not. SILAC proteomics and cell assays revealed that 3 promoted tau degradation through an autophagic mechanism, which was in contrast to that of other tau-lowering compounds previously identified by our group.
CONCLUSIONS:
During the course of structure-activity relationship (SAR) development, we prepared compound 13 by acid-catalyzed dehydration of 1. 13 had undergone an unexpected structural rearrangement through the isoMyricanol substitution pattern (e.g., 16), as verified by X-ray structural analysis. Compound 13 displayed robust tau-lowering activity, and, importantly, its enantiomers reduced tau levels similarly. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)