| In vitro: |
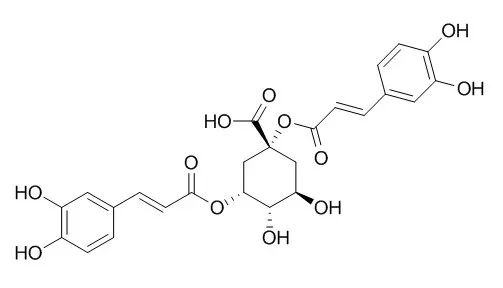
| Zhongguo Zhong Yao Za Zhi. 2014 Jun;39(12):2275-80. | | Regulation of syringin, chlorogenic acid and 1,5-dicaffeoylquinic acid biosynthesis in cell suspension cultures of Saussurea involucrata.[Pubmed: 25244758] | Syringin, chlorogenic acid and 1,5-dicaffeoylquinic acid are three main bioactive ingredients in herbs of Saussurea involucrata with various pharmacological properties, while their contents are very low.
METHODS AND RESULTS:
In this study, the biosynthesis of syringin, chlorogenic acid and 1,5-dicaffeoylquinic acid in the cell suspension cultures of S. involucrata were regulated by feeding carbon sources and precursors, which resulted in a great increase of the contents and yields of the above three bioactive ingredients. After 16 days of fermentation, the yields of syringin, chlorogenic acid and 1,5-dicaffeoylquinic acid reached 339.0, 225.3, 512.7 mg x L(-1), respectively. Meanwhile, their contents increased up to 67.9, 1.9, 10.6 times of wild medicinal material, respectively.
CONCLUSIONS:
The results provided a solid basis for further studies on application of cell suspension cultures of S. involucrata for large-scale production of bioactive compounds syringin, chlorogenic acid and 1,5-dicaffeoylquinic acid. | | Organic. Chem., 1999, 72. | | 1,5-Dicaffeoylquinic acid, an antioxidant component of Cynara cardunculus leaves[Reference: WebLink] |
METHODS AND RESULTS:
This study describes the isolation, identification and some antioxidant properties of 1,5-Dicaffeoylquinic acid. This compound was isolated from a methanolic extract of Cynara cardunculus leaves. The isolation was based on a selective extraction of 1,5-Dicaffeoylquinic acid with diethylether from a weak acidic water-methanolic solution and on column chromatography. The compound structure was determined by 1D and 2D H-NMR spectroscopy. Inhibition of hemolysis induced by hydrogen peroxide and the anti-radical effect of both 1,5-Dicaffeoylquinic acid and cynarin (1,3-Dicaffeoylquinic acid) were studied. Cynarin was formed from 1,5-Dicaffeoylquinic acid by trans-esterification during solvent extraction.
CONCLUSIONS:
The antioxidant activity of dicaffeoylquinic acids in both systems was found to be stronger than that of ascorbic acid. | | Brain Res. 2010 Aug 6;1347:142-8. | | 1, 5-Dicaffeoylquinic acid-mediated glutathione synthesis through activation of Nrf2 protects against OGD/reperfusion-induced oxidative stress in astrocytes.[Pubmed: 20513363 ] | Oxidative stress plays an important role in pathological processes of cerebral ischemia followed by reperfusion.
METHODS AND RESULTS:
The effect of 1,5-Dicaffeoylquinic acid (1, 5-diCQA) on primary culture rat cortical astrocytes induced by oxygen and glucose deprivation (OGD)/reperfusion was evaluated in this study. Appropriate concentration of 1, 5-diCQA pretreatment significantly suppressed cell death, reduced the production of reactive oxygen species, prevented glutathione (GSH) depletion, increased the activity of glutamate-cysteine ligase (GCL), and triggered Nrf2 nuclear translocation in astrocytes induced by 4h of OGD and 20 h of reperfusion. Interestingly, these protective effects were greatly attenuated in Nrf2 siRNA-transfected cells.
CONCLUSIONS:
We conclude that 1, 5-diCQA has antioxidant signaling properties that upregulate GSH synthesis by stimulating the Nrf2 pathway in astrocytes and protects them from cell death in an in vitro model of ischemia/reperfusion. | | J Enzyme Inhib Med Chem . 2016;31(2):266-75. | | Antioxidant, antiradical, and anticholinergic properties of cynarin purified from the Illyrian thistle (Onopordum illyricum L.)[Pubmed: 25792498] | | Abstract
Cynarin is a derivative of hydroxycinnamic acid and it has biologically active functional groups constituent of some plants and food. We elucidated the antioxidant activity of cynarin by using different in vitro condition bioanalytical antioxidant assays like DMPD(•+), ABTS(•+), O2(•-), DPPH(•) and H2O2 scavenging effects, the total antioxidant influence, reducing capabilities, Fe(2+) chelating and anticholinergic activities. Cynarin demonstrated 87.72% inhibition of linoleic acid lipid peroxidation at 30 μg/mL concentration. Conversely, some standard antioxidants like trolox, α-tocopherol, butylated hydroxytoluene (BHT), and butylated hydroxyanisole (BHA) exhibited inhibitions of 90.32, 75.26, 97.61, 87.30%, and opponent peroxidation of linoleic acid emulsion at the identical concentration, seriatim. Also, cynarin exhibited effective DMPD(•+), ABTS(•+), O2(•-), DPPH(•), and H2O2 scavenging effects, reducing capabilities and Fe(2+) chelating effects. On the contrary, IC50 and K(i) parameters of cynarin for acetylcholinesterase enzyme inhibition were determined as 243.67 nM (r(2): 0.9444) and 39.34 ± 13.88 nM, respectively. This study clearly showed that cynarin had marked antioxidant, anticholinergic, reducing ability, radical-scavenging, and metal-binding activities.
Keywords: Antiacetylcholinesterase; Onopordum illyricum; anticholinergic; antioxidant activity; cynarin; radical scavenging. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)