| Structure Identification: |
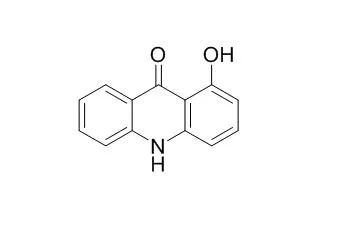
| Bioorg Med Chem. 2008 Jan 1;16(1):313-21. | | SAR of a series of anti-HSV-1 acridone derivatives, and a rational acridone-based design of a new anti-HSV-1 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridine series.[Pubmed: 17937990] | Herpes Simplex Virus (HSV) infections are among the most common human diseases. In this work, we assess the structural features and electronic properties of a series of ten 1-Hydroxyacridone derivatives (1a-j) recently described as a new class of non-nucleoside inhibitors of Herpes Simplex Virus-1 (HSV-1).
METHODS AND RESULTS:
Based on these molecules, we applied rigid analogue and isosteric replacement approaches to design and synthesize nine new 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridine derivatives (2a-i). The biological and computational results of these new molecules were compared with 1-Hydroxyacridones. An inhibitory profile was observed in 10-Cl substituted 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridine derivative (2f), which presents the same substituent at the analogous position of 1-Hydroxyacridone derivative (1b).
CONCLUSIONS:
The structure-activity relationship (SAR) studies pointed out the 10-position next to nitrogen atom as important for the anti-HSV-1 profile in the pyrazolo-naphthyridine derivatives tested, which reinforced the promising profile for further experimental investigation.
The most potent acridone and pyrazolo-naphthridine derivatives were also submitted to an in silico ADMET screening in order to determine their overall drug-score, which confirmed their potential antiviral profile. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)