| Description: |
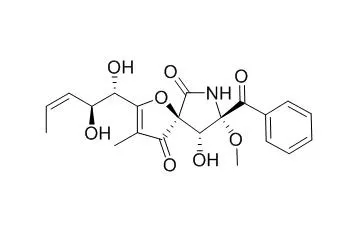
14-Norpseurotin A shows good antileishmanial and moderate anticancer activities. It also displays significant antimicrobial activities against Escherichia coli, Bacillus subtilis, and Micrococcus lysoleikticus with MICs of 3.74, 14.97, and 7.49 microM , respectively. 14-Norpseurotin A can significantly induce neurite outgrowth of rat pheochromocytoma cells (PC12) at a 10.0 microM concentration. |
| In vitro: |
| Nat Prod Commun. 2012 Feb;7(2):165-8. | | Antiparasitic and anticancer constituents of the endophytic fungus Aspergillus sp. strain F1544.[Pubmed: 22474943] |
METHODS AND RESULTS:
With the combined goal of finding the best anti-parasitic and anti-cancer activities as well as isolating the bioactive agents and studying their structures and biological properties, we proceeded to perform a small-scale cultivation of Aspergillus sp. strain F1544 using Potato Dextrose, Malt Extract, Czapek Dox and Eight Vegetables media. From the more promising extracts (obtaining using potato dextrose and czapek dox media in large scale) of this fungus, we isolated the five compounds: pseurotin A (1), 14-Norpseurotin A (2), FD-838 (3), and pseurotin D (4), and fumoquinone B (5).
METHODS AND RESULTS:
All compounds showed good antileishmanial and moderate anticancer activities. | | J Nat Prod. 2008 Jun;71(6):985-9. | | Cytotoxic alkaloids and antibiotic nordammarane triterpenoids from the marine-derived fungus Aspergillus sydowi.[Pubmed: 18505285 ] |
METHODS AND RESULTS:
Three new diketopiperazine alkaloids, 6-methoxyspirotryprostatin B (1), 18-oxotryprostatin A (2), and 14-hydroxyterezine D (3), with an oxaspiro[4.4]lactam moiety, 14-Norpseurotin A (4), and the 29-nordammarane triterpenoid 6beta,16beta-diacetoxy-25-hydroxy-3,7-dioxy-29-nordammara-1,17(20)-dien-21-oic acid (5), as well as 12 known compounds (6- 17), were isolated from the ethyl acetate extract of a marine-derived fungal strain, Aspergillus sydowi PFW1-13. The structures of compounds 1- 5 were elucidated by comprehensive spectroscopic analysis.
CONCLUSIONS:
Compounds 1- 3 exhibit weak cytotoxicity against A-549 cells, with IC 50 values of 8.29, 1.28, and 7.31 microM, respectively. Compound 1 also shows slight cytotoxicity against HL-60 cells, with an IC 50 value of 9.71 microM. Compounds 4 and 5 display significant antimicrobial activities against Escherichia coli, Bacillus subtilis, and Micrococcus lysoleikticus with MICs of 3.74, 14.97, and 7.49 microM and 10.65, 5.33, and 10.65 microM, respectively. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)