| In vitro: |
| Sex Transm Dis. 2011 Feb;38(2):82-8 | | Antimicrobial activity of flavonoids from Piper lanceaefolium and other Colombian medicinal plants against antibiotic susceptible and resistant strains of Neisseria gonorrhoeae.[Pubmed: 20921932 ] | The successful treatment of Neisseria gonorrhoeae (NG) infections is increasingly problematic because of the resistance of this pathogen to multiple antimicrobial agents. This development underscores the need for new antimicrobial sources. In the current study, 21 crude methanol extracts, from 19 plants used in Colombian traditional medicine for cutaneous infections, were screened for antimicrobial activity against NG.
METHODS AND RESULTS:
Extracts were screened by disc susceptibility assay. In addition, the minimum inhibitory concentrations of active compounds from P. lanceaefolium were assayed using a panel of 26 NG strains comprising 12 antibiotic-resistant phenotypes.
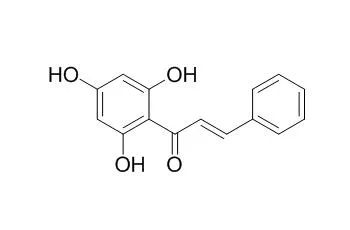
In all, 71% of the crude extracts exhibited antibacterial activity against the antibiotic susceptible NG strain WHO V, whereas 10% of the extracts inhibited penicillinase-producing NG strain GC1-182. The crude extract of Piper lanceaefolium was the only extract to show significant activity without ultraviolet (UV) light activation. Preliminary screening identified 3 compounds in this plant possessing antimicrobial activity: the flavonoids 5,7-dihydroxyflavanone (pinocembrin), 2',4',6'-trihydroxychalcone (Pinocembrin chalcone), and the prenylated benzoic acid derivative cyclolanceaefolic acid methyl ester. Pinocembrin and Pinocembrin chalcone inhibited 100% of the NG panel at 64 μg/mL and 128 μg/mL, respectively, whereas cyclolanceaefolic acid methyl ester inhibited 44% of the strains at 128 μg/mL.
CONCLUSIONS:
This is the first report of the antibacterial activity of Columbian plants against NG. The activity of the 2 flavonoids, pinocembrin, and Pinocembrin chalcone, toward both susceptible and resistant NG strains makes them promising candidates for further research. | | Bioorg Med Chem. 2007 Mar 15;15(6):2396-402. | | Synthesis and evaluation of 2',4',6'-trihydroxychalcones as a new class of tyrosinase inhibitors.[Pubmed: 17267225 ] |
METHODS AND RESULTS:
Bioactivity-guided fractionation of a methanol extract from the leaves of Piper lanceaefolium resulted in the isolation of four new benzoic acid derivatives (1-4), together with taboganic acid, pinocembrin, and Pinocembrin chalcone.
CONCLUSIONS:
Lanceaefolic acid methyl ester (3) and Pinocembrin chalcone displayed activity against Candida albicans with a minimal inhibitory concentration value of 100 microg/mL in both cases. | | J Agric Food Chem. 2001 Jun;49(6):3046-50. | | Structural analysis of a novel antimutagenic compound, 4-Hydroxypanduratin A, and the antimutagenic activity of flavonoids in a Thai spice, fingerroot (Boesenbergia pandurata Schult.) against mutagenic heterocyclic amines.[Pubmed: 11410007] |
METHODS AND RESULTS:
Six compounds were isolated from fresh rhizomes of fingerroot (Boesenbergia pandurata Schult.) as strong antimutagens toward 3-amino-1,4-dimethyl-5H-pyrido[4,3-b]indole (Trp-P-1) in Salmonella typhimurium TA98. These compounds were 2',4',6'-trihydroxychalcone (Pinocembrin chalcone; 1), 2',4'-dihydroxy-6'-methoxychalcone (cardamonin; 2), 5,7-dihydroxyflavanone (pinocembrin; 3), 5-hydroxy-7-methoxyflavanone (pinostrobin; 4), (2,4,6-trihydroxyphenyl)-[3'-methyl-2'-(3' '-methylbut-2' '-enyl)-6'-phenylcyclohex-3'-enyl]methanone (5), and (2,6-dihydroxy-4-methoxyphenyl)-[3'-methyl-2'-(3' '-methylbut-2' '-enyl)-6'-phenylcyclohex-3'-enyl]methanone (panduratin A; 6). Compound 5 was a novel compound (tentatively termed 4-hydroxypanduratin A), and 1 was not previously reported in this plant, whereas 2-4 and 6 were known compounds.
CONCLUSIONS:
The antimutagenic IC(50) values of compounds 1-6 were 5.2 +/- 0.4, 5.9 +/- 0.7, 6.9 +/- 0.8, 5.3 +/- 1.0, 12.7 +/- 0.7, and 12.1 +/- 0.8 microM in the preincubation mixture, respectively. They also similarly inhibited the mutagenicity of 3-amino-1-methyl-5H-pyrido[4,3-b]indole (Trp-P-2) and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). All of them strongly inhibited the N-hydroxylation of Trp-P-2. Thus, the antimutagenic effect of compounds 1-6 was mainly due to the inhibition of the first step of enzymatic activation of heterocyclic amines. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)