| In vitro: |
| Int J Mol Sci. 2013 Sep 18;14(9):19186-201. | | Cinnamomum cassia essential oil inhibits α-MSH-induced melanin production and oxidative stress in murine B16 melanoma cells.[Pubmed: 24051402] | Essential oils extracted from aromatic plants exhibit important biological activities and have become increasingly important for the development of aromatherapy for complementary and alternative medicine. The essential oil extracted from Cinnamomum cassia Presl (CC-EO) has various functional properties; however, little information is available regarding its anti-tyrosinase and anti-melanogenic activities.
METHODS AND RESULTS:
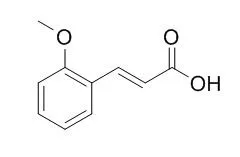
In this study, 16 compounds in the CC-EO have been identified; the major components of this oil are cis-2-Methoxycinnamic acid (43.06%) and cinnamaldehyde (42.37%). CC-EO and cinnamaldehyde exhibited anti-tyrosinase activities; however, cis-2-Methoxycinnamic acid did not demonstrate tyrosinase inhibitory activity. In murine B16 melanoma cells stimulated with α-melanocyte-stimulating hormone (α-MSH), CC-EO and cinnamaldehyde not only reduced the melanin content and tyrosinase activity of the cells but also down-regulated tyrosinase expression without exhibiting cytotoxicity. Moreover, CC-EO and cinnamaldehyde decreased thiobarbituric acid-reactive substance (TBARS) levels and restored glutathione (GSH) and catalase activity in the α-MSH-stimulated B16 cells.
CONCLUSIONS:
These results demonstrate that CC-EO and its major component, cinnamaldehyde, possess potent anti-tyrosinase and anti-melanogenic activities that are coupled with antioxidant properties. Therefore, CC-EO may be a good source of skin-whitening agents and may have potential as an antioxidant in the future development of complementary and alternative medicine-based aromatherapy. | | J Agric Food Chem. 2001 Oct;49(10):4656-61. | | Selective growth inhibitor toward human intestinal bacteria derived from Pulsatilla cernua root.[Pubmed: 11600003] | Among 21 medicinal plants, the growth-inhibiting activity of Pulsatilla cernua root-derived materials toward human intestinal bacteria was examined by using an impregnated paper disk method.
METHODS AND RESULTS:
The biologically active components of P. cernua roots were characterized as 4-hydroxy-3-methoxycinnamic acid and 3,4-dihydroxycinnamic acid by spectroscopic analysis. The activity was compared with that of six commercially available cinnamic acid derivatives trans-cinnamaldehyde, trans-cinnamic acid, cinnamyl alcohol, 2-Methoxycinnamic acid, 3-methoxycinnamic acid, and 4-methoxycinnamic acid. The growth responses varied with each bacterial strain tested. Two isolated compounds revealed a potent inhibition against Clostridium perfringens, and moderate to weak activity against Escherichia coli was exhibited by 4-hydroxy-3-methoxycinnamic acid. Weak or no inhibitory activity was obtained against the bifidobacteria or Lactobacillus acidophilus. The inhibitory effect was much more pronounced in C. perfringens and E. coli as compared to B. adolescentis, B. bifidum, B. fragilis, B. longum, or L. acidophilus. Cinnamaldehyde exhibited a strong growth-inhibiting activity, but no inhibition was observed from treatments with trans-cinnamic acid, cinnamyl alcohol, 2-Methoxycinnamic acid, 3-methoxycinnamic acid, and 4-methoxycinnamic acid.
METHODS AND RESULTS:
These results may be an indication of at least one of the pharmacological actions of P. cernua root. | | US 8758864 B2[P]. 2014. | | Photosensitive semiconductor nanocrystals, photosensitive composition comprising semiconductor nanocrystals and method for forming semiconductor nanocrystal pattern using the same.[Reference: WebLink] | | 4. The organic-inorganic hybrid electroluminescent device according to claim 1, wherein the compound containing a photosensitive functional group is selected from a group consisting of methacrylic acid, crotonic acid, vinylacetic acid, tiglic acid, 3,3-dimethylacrylic acid, trans-2-pentenoic acid, 4-pentenoic acid, trans-2-methyl-2-pentenoic acid, 2,2-dimethyl-4-pentenoic acid, trans-2-hexenoic acid, trans-3-hexenoic acid, 2-ethyl-2-hexenoic acid, 6-heptenoic acid, 2-octenoic acid, citronellic acid, undecylenic acid, myristoleic acid, palmitoleic acid, oleic acid, elaidic acid, cis-11-elcosenoic acid, euric acid, nervonic acid, trans-2,4-pentadienoic acid, 2,4-hexadienoic acid, 2,6-heptadienoic acid, geranic acid, linoleic acid, 11,14-eicosadienoic acid, cis-8,11,14-eicosatrienoic acid, arachidonic acid, cis-5,8,11,14,17-eicosapentaenoic acid, cis-4,7,10,13,16,19-docosahexaenoic acid, fumaric acid, maleic acid, itaconic acid, ciraconic acid, mesaconic acid, trans-glutaconic acid, trans-beta-hydromuconic acid, trans-traumatic acid, trans-muconic acid, cis-aconitic acid, trans-aconitic acid, cis-3-chloroacrylic acid, trans-3-chloroacrylic acid, 2-bromoacrylic acid, 2-(trifluoromethyl)acryl-ic acid, trans-styrylacetic acid, trans-cinnamic acid, alpha.-methylcinnamic acid, 2-methylcinnamic acid, 2-fluorocinnamic acid, 2-(trifluoromethyl)cinnamic acid, 2-chlorocinnamic acid, 2-Methoxycinnamic acid, 2-hydroxycinnamic acid, 2-nitrocinnamic acid, 2-carboxycinnamic acid, trans-3-fluorocinnamic acid, 3-(trifluoromethyl)cinnamic acid, 3-chlorocinnamic acid, 3-bromocinnamic acid, 3-methoxycinnamic acid, 3-hydroxycinnamic acid, 3-nitrocinnamic acid, 4-Methylcinnamic acid, 4-fluorocinnamic acid, trans-4-(trifluoromethyl)-cinnamic acid, 4-Chlorocinnamic acid, 4-bromocinnamic acid, 4-methoxycinnamic acid, 4-hydroxycinnamic acid, 4-nitrocinnamic acid, 3,3-dimethoxycinnamic acid, 4-vinylbenzoic acid, allyl methyl sulfide, allyl disulfide, diallyl amine, oleylamine, 3-amino-1-propanol vinyl ether, 4-chlorocinnamonitrile, 4-methoxycinnamonitrile, 3,4-dimethoxycinnamonitrile, 4-dimethylaminocinnamonitrile, acrylonitrile, allyl cyanide, crotononitrile, methacrylonitrile, cis-2-pentenenitrile, trans-3-pentenenitrile, 3,7-dimethyl-2,6-octadienenitrile, and 1,4-dicyano-2-butene. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)