| Kinase Assay: |
| Biological & Pharmaceutical Bulletin, 1999, 22(7):667-673. | | Biochemical Characterization of 60S Acidic Ribosomal P Proteins Associated with CK-II from Bamboo Shoots and Potent Inhibitors of Their Phosphorylation in Vitro.[Reference: WebLink] |
METHODS AND RESULTS:
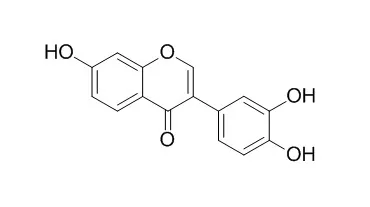
Three effective phosphate acceptors (35, 15 and 13 kDa polypeptides) for casein kinase II (CK-II) in the Superdex CK-II fraction prepared from a 0.5 M NaCl extract of bamboo shoots were selectively purified by glycyrrhizin (GL)-affinity column chromatography (HPLC). These three proteins (p35, p15 and p13) were identified as 60S acidic ribosomal P proteins by determination of their partial N-terminal sequences. CK-II was associated with p35 since the GL-affinity fraction was coprecipitated with an anti-serum against Drosophila CK-IIbeta. Moreover, a derivative (oGA) of glycyrrhetinic acid (GA) and several polyphenol-containing anti-oxidative compounds [quercetin, epigallocatechin gallate (EGCG) and two isoflavones, i.e., 3',4',7-Trihydroxyisoflavone (3',4',7-THI) and 8-chloro-3',4',5,7-tetrahydroxyisoflavone (8C-3',4',5,7-THI)] inhibited the CK-II-mediated phosphorylation of 60S acidic ribosomal P proteins in vitro. Quercetin was found to be the most effective compound on CK-II activity since its ID50 was approx. 50 nM.
CONCLUSIONS:
These results suggest that (i) GL-affinity column chromatography is useful for the selective purification of 60S acidic ribosomal P proteins as a heterocomplex associated with CK-II from various cell sources; (ii) natural anti-oxidative compounds with polyphenols, but not GL and GA, act as potent CK-II suppressors; and (iii) CK-II mediates the regulation of the physiological functions of 60S acidic ribosomal P proteins in growing plant cells. | | The Journal of Biological Chemistry, 05 May 2010, 285(28):21458-21466. | | 7,3',4'-Trihydroxyisoflavone inhibits epidermal growth factor-induced proliferation and transformation of JB6 P+ mouse epidermal cells by suppressing cyclin-dependent kinases and phosphatidylinositol 3-kinase.[Reference: WebLink] | Numerous in vitro and in vivo studies have shown that isoflavones exhibit anti-proliferative activity against epidermal growth factor (EGF) receptor-positive malignancies of the breast, colon, skin, and prostate. 7,3',4'-Trihydroxyisoflavone (3',4',7-Trihydroxyisoflavone, 7,3',4'-THIF) is one of the metabolites of daidzein, a well known soy isoflavone, but its chemopreventive activity and the underlying molecular mechanisms are poorly understood.
METHODS AND RESULTS:
In this study, 7,3',4'-THIF prevented EGF-induced neoplastic transformation and proliferation of JB6 P mouse epidermal cells. It significantly blocked cell cycle progression of EGF-stimulated cells at the G(1) phase. As shown by Western blot, 7,3',4'-THIF suppressed the phosphorylation of retinoblastoma protein at Ser-795 and Ser-807/Ser-811, which are the specific sites of phosphorylation by cyclin-dependent kinase (CDK) 4. It also inhibited the expression of G(1) phase-regulatory proteins, including cyclin D1, CDK4, cyclin E, and CDK2. In addition to regulating the expression of cell cycle-regulatory proteins, 7,3',4'-THIF bound to CDK4 and CDK2 and strongly inhibited their kinase activities. It also bound to phosphatidylinositol 3-kinase (PI3K), strongly inhibiting its kinase activity and thereby suppressing the Akt/GSK-3beta/AP-1 pathway and subsequently attenuating the expression of cyclin D1.
CONCLUSIONS:
Collectively, these results suggest that CDKs and PI3K are the primary molecular targets of 7,3',4'-THIF in the suppression of EGF-induced cell proliferation. These insights into the biological actions of 7,3',4'-THIF provide a molecular basis for the possible development of new chemoprotective agents. |
|
| Structure Identification: |
| The Journal of Antibiotics, 01 Sep 1989, 42(9):1344-1349. | | Isolation of isoflavonoids possessing antioxidant activity from the fermentation broth of Streptomyces sp.[Reference: WebLink] |
METHODS AND RESULTS:
Three antioxidant isoflavonoids characterized as 4',7,8-trihydroxyisoflavone (1), 3',4',7-Trihydroxyisoflavone (2) and 8-chloro-3',4',5,7-tetrahydroxyisoflavone (3) were isolated from the cultured broth of Streptomyces sp. OH-1049. Among them, 3 is a novel isoflavonoid possessing a chlorine atom in the molecule.
CONCLUSIONS:
In in vitro studies, these antibiotics were found to possess antioxidant activity whereas showed almost no cytocidal activities against HeLa S3 cells. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)